Amide Formation from Acid Halides
Amide formation from Acid halides
Acid halides also called acyl halides are derivatives of carboxylic acids in which the -OH group of carboxylic acid is replaced by halogen.
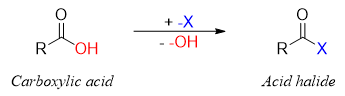
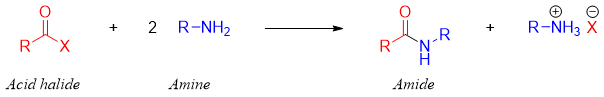
Amides are synthesized from acid halides by reacting acid halide with ammonia or amines. The general reaction for the preparation of amides is shown below.
Mechanism:
For the synthesis of amides from acid halides, the acid halide is reacted with two equivalents of ammonia or amines. The first equivalent amine reacts with acyl halide while, the second equivalent reacts with the proton produced during the reaction.
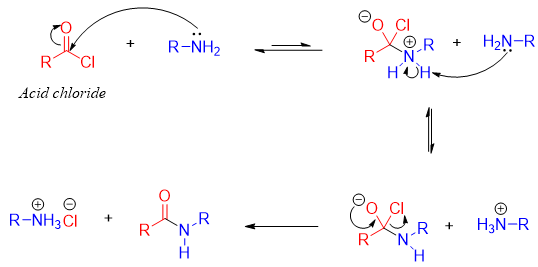
Once protonated, the second equivalent amine no more acts as a nucleophile therefore, it does not react with acyl halide. For this purpose, the amine is used in excess (2 equivalents) to make sure there is enough free amine to react with acyl halide.
The second equivalent amine can also be protonated by the HCl produced during the reaction. The mechanism for the production of HCl is shown below.
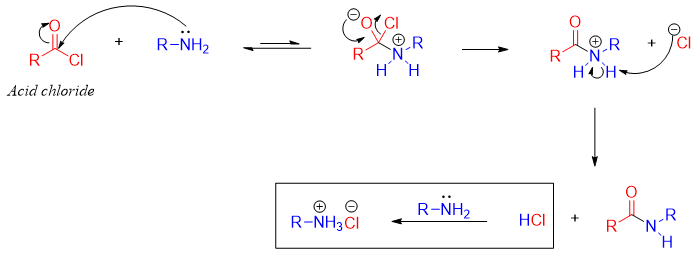
For the synthesis of amides from acyl halide only ammonia, monosubstituted and disubstituted amines can be used. Tertiary amines can not be used for the synthesis of amides from acyl halides.
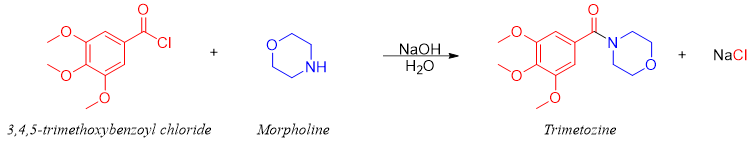
If the amine is expensive and valuable, then inexpensive base like NaOH is used along with 1 equivalent of amine. For example, Trimetozine (sedative drug) is prepared by reacting 1 mole of morpholine (an amine) with 1 mole of 3,4,5-trimethoxybenzoyl chloride (acid halide) in the presence of 1 mole of NaOH.