Amide Formation Using DCC
Amide formation using DCC
The synthesis of amides from carboxylic acids is not an easy approach. This is due to the acid base reaction between the acid and amines. Amines being basic in nature deprotaonates the carboxylic acid converting it to carboxylate which is less reactive towards nucleophilic substitution reactions.
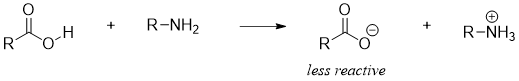
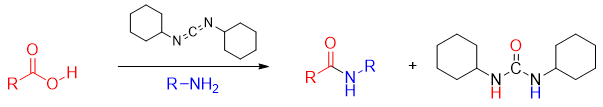
For this purpose, the -OH group of carboxylic acid is often converted to a good nonacidic leaving group. One very useful technique employed in the synthesis of amides from carboxylic acids is the use of coupling agent called dicyclohexylcarbodiimide (DCC). DCC basically activates carboxylic acids. DCC is frequently used in the synthesis of small proteins or peptides.
Mechanism:
Initially, the protonation of DCC by carboxylic acid takes place.
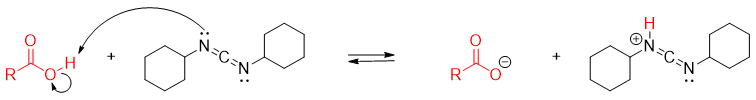
Once the carboxylate ion is formed it adds to protonated DCC to generate reactive acylating agent.
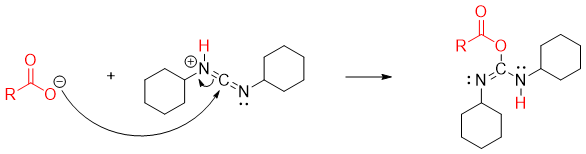
The amines attacks on the carbonyl carbon to form tetrahedral intermediate.
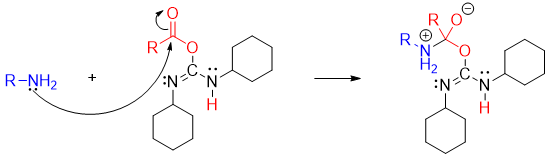
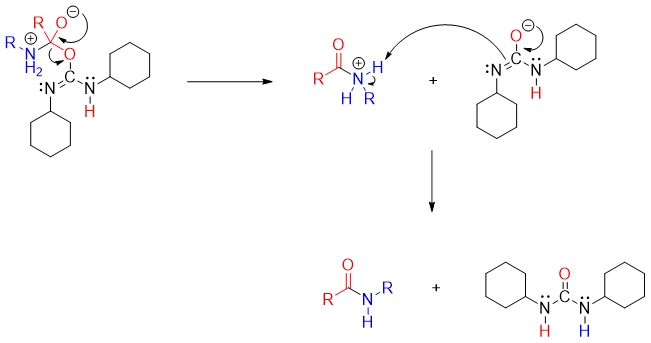
The tetrahedral intermediate loses dicyclohexylurea to produce amide.
Use of DCC in polypeptide synthesis:
The synthesis of polypeptide is difficult due to two functional groups (-NH2 and -COOH) that combines in different ways resulting in synthesis of polypeptides with random amino acid sequences. For instance, if one wants to synthesize Ala-Gly dipeptide then Ala-Gly is one of four possible products. Other three are Ala-Ala, Gly-Gly, and Gly-Ala. To solely synthesis Ala-Gly a four step strategy is applied.
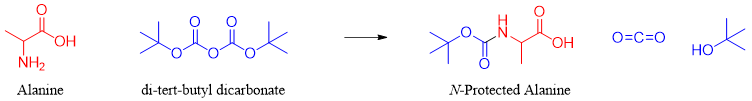
1) Protecting the amino group of Ala:
First the -NH2 group of Ala is protected by using different protecting groups. The most common protecting group used is di-tert-butyl dicarbonate commonly referred as Boc anhydride. The protection of amine group insures it will not act as a nucleophile anymore.
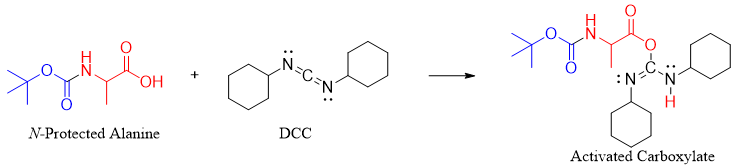
2) Activating carboxyl group of N-protected Ala by DCC
The N-protected Ala is reacted with DCC to activate the carboxyl group as the DCC acts a good leaving group.
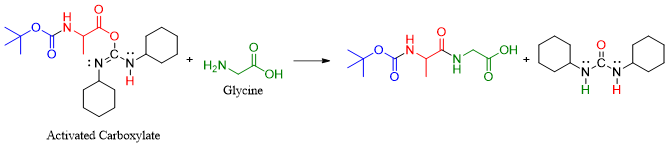
3) Adding Glycine as the second amino acid
Once the -NH2 group of Alanine is protected, and the carboxylate group is activated the second amino acid Glycine is added. The amino group of Glycine will act as nucleophile and will add to the activated carboxylate group of Alanine.
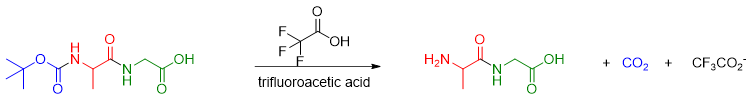
4) Deprotection of Alanine amino acid
Once the Glycine is successfully added, the Alanine is deprotected by adding trifluoroacetic acid.