Hydrolysis of Amides
Hydrolysis of Amides
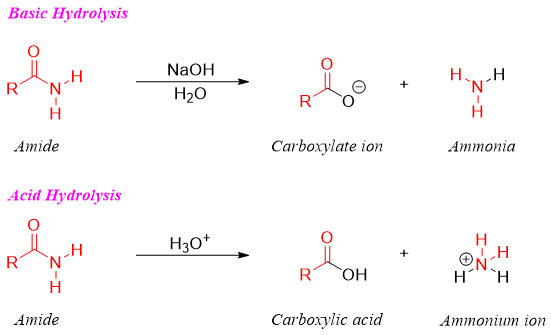
Hydrolysis of amides produces carboxylic acids under both basic and acidic conditions. Amides being stable derivatives of carboxylic acids require stronger conditions to hydrolyze thus, it is heated either with 40% NaOH (aqueous) or 6M HCl to transform into carboxylic acids.
Mechanism of base promoted hydrolysis of an amide:
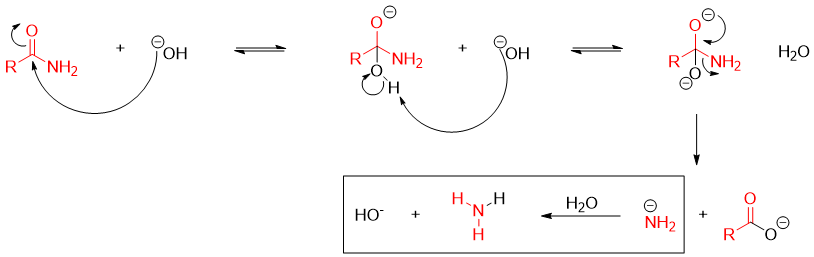
Hydroxide ion being stronger nucleophile than water adds to the carbonyl group and generates tetrahedral intermediate. The first tetrahedral intermediate formed has two potential leaving groups, -OH and -NH2. -OH being weaker base than -NH2 has more chances to eliminate thus, regenerating the amide. Therefore, the second hydroxide ion (base) abstracts proton from -OH and generates second tetrahedral intermediate with two -O- groups. -NH2 being weaker base than -O- is eliminated resulting in the formation of carboxylate ion. The NH2- being stronger base abstracts proton from water and forms ammonia and HO- ion. In this reaction the first equivalent of -OH is not regenerated while the second equivalent is generated. Therefore, only one mole of -OH acts as a catalyst.
Mechanism of acid catalyzed hydrolysis of an amide:
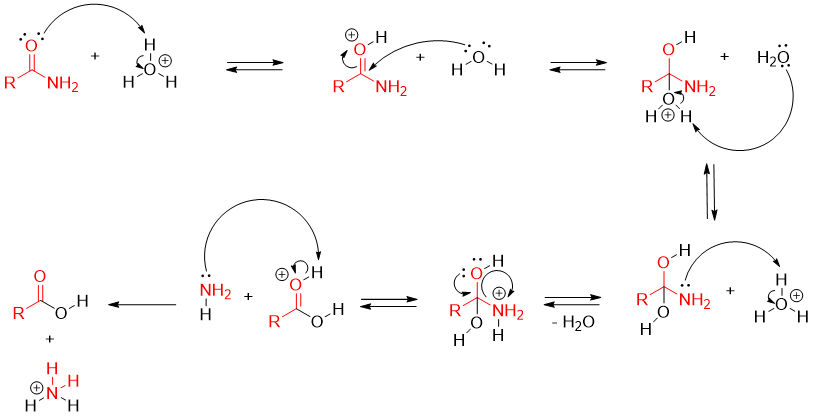
In acid catalyzed hydrolysis of amides, the carbonyl oxygen is protonated first. This protonation increases the electrophilic character of carbonyl carbon hence, making it more susceptible for nucleophilic attack. Addition of water molecule generates first tetrahedral intermediate. This intermediate is in equilibrium with non-protonated tetrahedral intermediate. The second non-protonated tetrahedral intermediate is protonated on nitrogen atom to generate a good leaving group ammonia. The neutral ammonia is released and is protonated into ammonium ion. This makes ammonia inactive to act as a nucleophile and stops the reaction to reverse.
