Dehydration of Amides to Nitriles
Dehydration of Amides to Nitriles
Amides are closely related to the nitriles. Amides are nitriles which have gain water molecule. Therefore, nitriles can be formed by removing water molecule from primary amides.
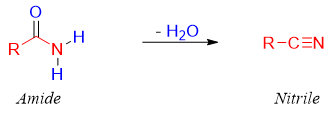
Nitrile functionalities are widely employed in organic synthesis and used in the synthesis of pharmaceuticals, agriculture chemicals and polymers. For this purpose, the conversion of an amide to nitrile is a useful transformation. Nitriles can be converted into carboxylic acids, ketones, amines, and large number of commercially significant molecules.
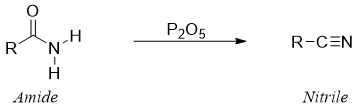
Most common method used to dehydrate amides to nitriles is using strong dehydration reagents. Phosphorous pentoxide (P2O5), phosphorous oxychloride (POCl3) and thionyl chloride (SOCl2) are generally used for this purpose.
Reaction of amide with P2O5:
Mechanism:
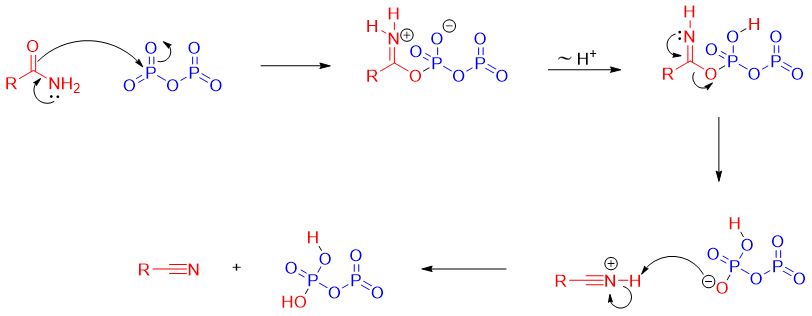
Reaction of amide with POCl3:
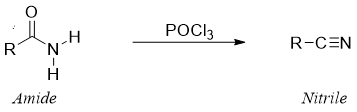
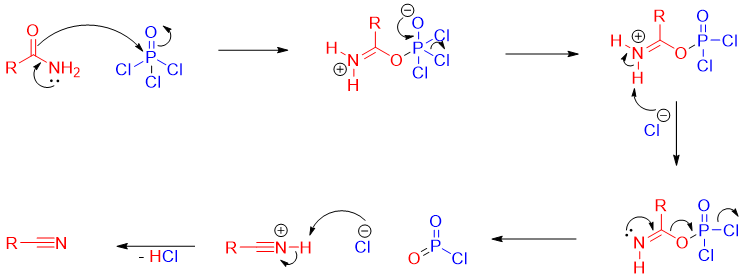
Mechanism:
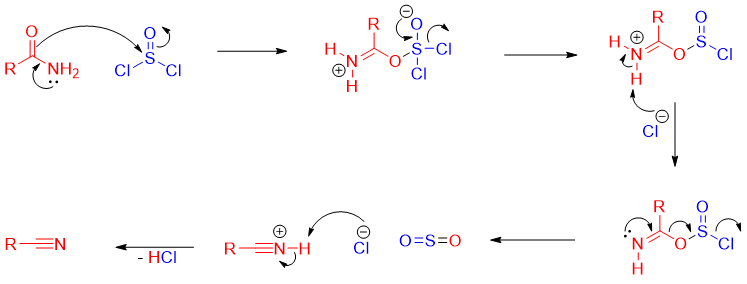
Reaction of amide with SOCl2:
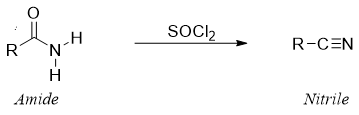
Mechanism: