Hofmann Rearrangement
Hofmann Rearrangement
Primary amines when reacted with Cl2 or Br2 in the presence of strong base forms amines with the elimination of carbonyl group. This reaction is called Hofmann rearrangement reaction.

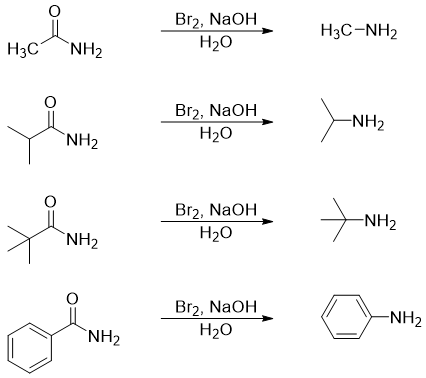
Using this method one can easily synthesize primary amines with primary, secondary, and tertiary alkyl groups or aryl group. For example
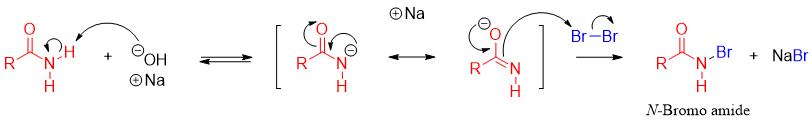
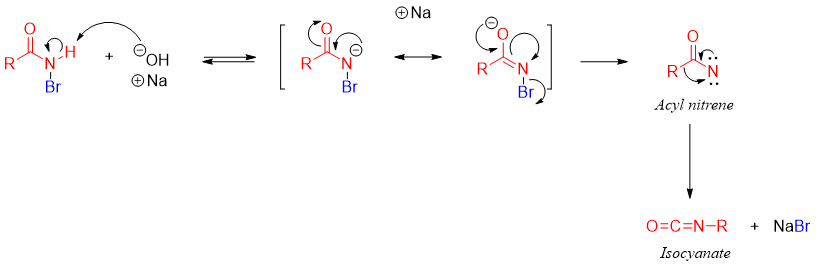
The mechanism of this reaction includes the formation of different intermediates. In the first step the slightly acidic proton of N-H is deprotonated by the base. The resulting de-protonated amide attacks on halogen molecule to form N-halo amide.
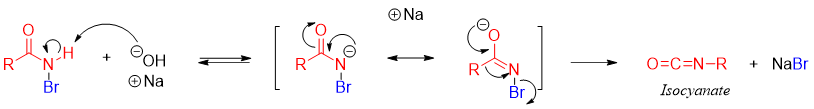
Next the second equivalent base abstracts proton from N-H of N-halo amide and the de-protonated N-halo amide rearrange to migrate the alkyl group and eliminate the halogen atom to form another intermediate called isocyanate.
The isocyanate formation can also take place via acyl nitrene intermediate.
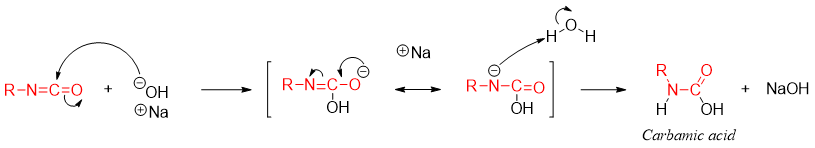
The third equivalent of base adds to the carbonyl carbon of isocyanate to form carbonimidate ion which reacts with water to give third intermediate called carbamic acid.
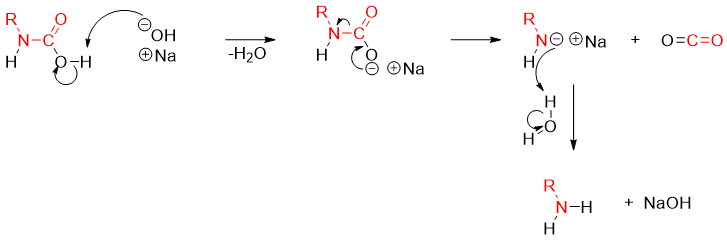
The carbamic acid is an unstable molecule hence it loses carbon dioxide gas spontaneously upon decarboxylation and produce corresponding amine.
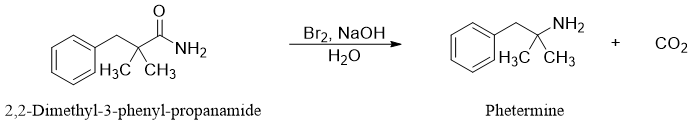
Hofmann rearrangement reaction often give high yields despite of complex mechanism. For example, Phentermine (appetite suppressant) is synthesized commercially from 2,2-Dimethyl-3-phenyl-propanamide via Hofmann rearrangement in high yields.