Amide Formation from Nitriles
Amide Formation from Nitriles
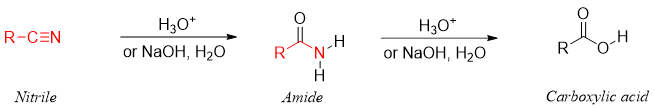
Nitriles are closely related to the amides. Nitriles are in fact primary amides which have lost water molecule. Therefore, primary amides can be formed by adding water molecule to nitriles.
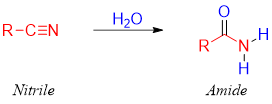
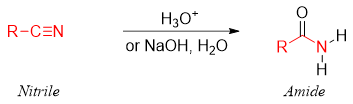
Primary amides are synthesized from nitriles by the acid or base catalyzed hydration of nitriles.
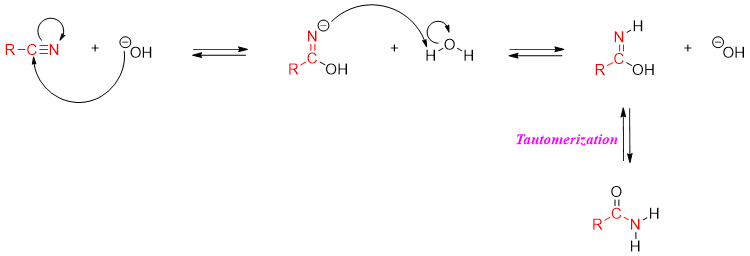
Following mechanism depicts base catalyzed hydration of nitrile to amide.
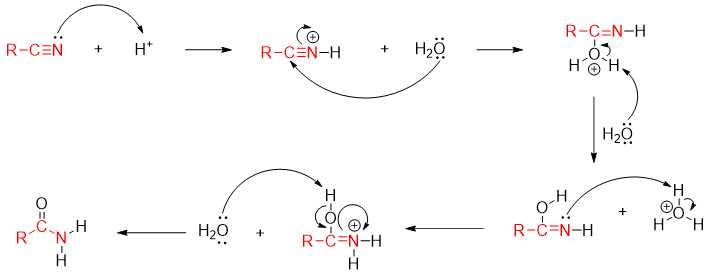
Mechanism of acid catalyzed hydration of nitrile to amide is shown below.
The amide formed from hydration of nitrile is further hydrolyzed to carboxylic acid. This conversion takes place both in acidic and basic medium.
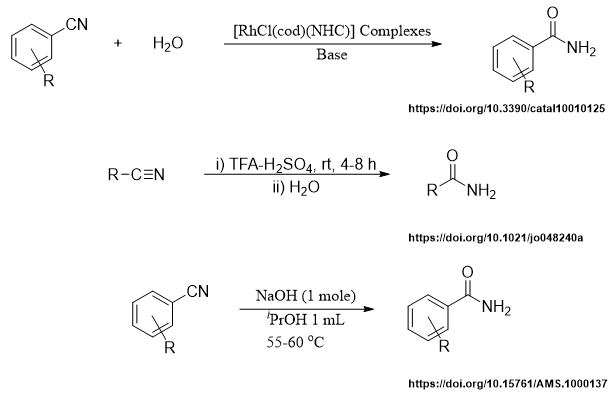
There are many reported methods for the selective hydration of nitriles to corresponding amides. Few are listed below.
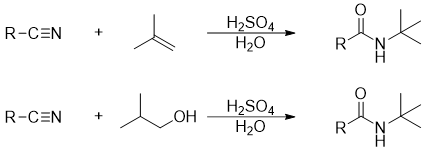
The Ritter Reaction:
In Ritter reaction nitriles are converted into secondary amides using different alkylating agents.
Mechanism:
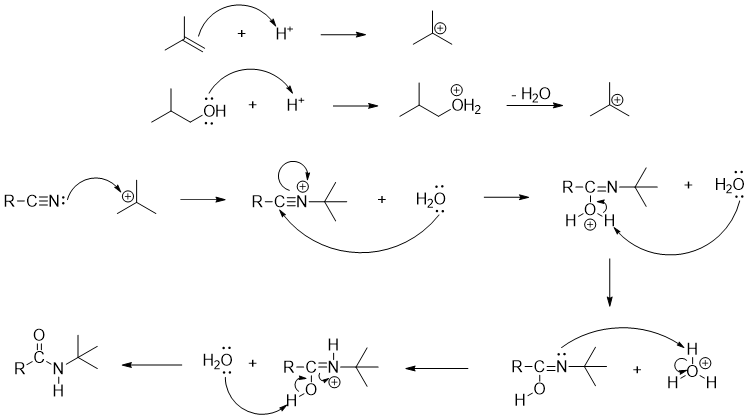
Those substrates which can produce a stable carbocation can be used as starting material for this reaction. Except benzylic alcohol primary alcohols cannot be used for this reaction.
