Protection of Alcohols
PROTECTION OF ALCOHOLS
There are instances that one functional group in a molecule interferes with an intended reaction on a second functional group elsewhere in the same molecule. One good example would be the preparation of Grignard reagent using a molecule having a halide group (-Cl, -Br, or –I) and hydroxyl (-OH) at the same time.
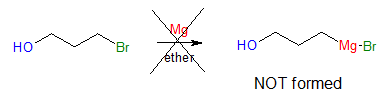
As observed, the above reaction will not occur since the formation of C―Mg is not compatible with the presence of acidic –OH group in the same molecule. To remedy this kind of situation, it would be advisable to introduce what we call “protecting groups” to the interfering functional group.
A protecting group or protective group is introduced into a molecule by chemically modifying a functional group to obtain a preferred outcome in a subsequent chemical reaction. This plays an important role in many multistep organic syntheses. In this article, we will focus on the methods used in protecting the alcohol functional group.
Protection, in general, involves three steps:
(1) introducing a protecting group to block the interfering functional group;
(2) carrying out the desired reaction; and
(3) removing the protecting group.
Chlorotrialkylsilanes as protecting groups (Cl―SiR3)
One of the common methods of alcohol protection is by reaction with a chloroalkylsilane, Cl―SiR3, to yield a trialkylether, R―O-SiR3. To be specific, chlorotrimethylsilane (Cl-TMS) is often used together with a base such as triethylamine. See an example reaction below.
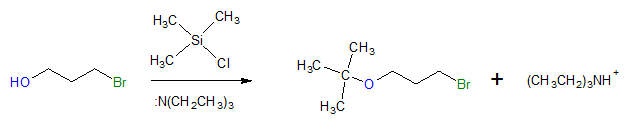
Mechanism:
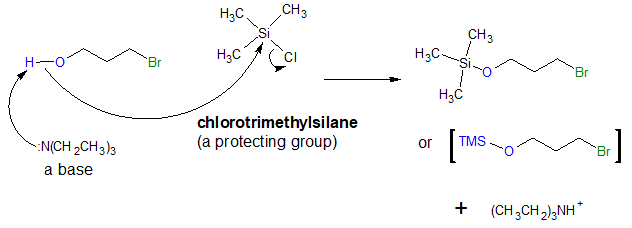
Once the –OH group is converted to a trimethylsilyl (TMS) ether, the desired subsequent step can then be carried out. Let’s say that the desired reaction is the formation of a Grignard reagent. With the introduction of protecting group, the reaction below will now proceed as written.
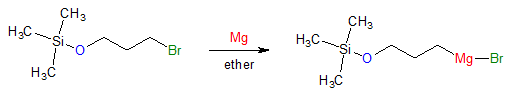
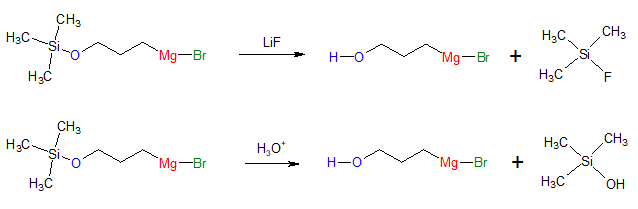
The nice thing about using protecting groups is that after carrying out the desired reaction, one can always remove the protecting group to revert back to the original alcohol group. This can be done by treatment with fluoride ions or through acid-catalyzed hydrolysis such as shown below:
Deprotection (removal of protecting group):
Sometimes, the trimethylsilyl group in the molecule is just represented as –TMS for simplification purposes. Just take note that whenever you encounter –TMS, it means that a protecting group is introduced in the molecule to protect the alcohol group.
