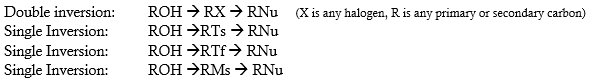Inversion Vs Double Inversion
INVERSION VERSUS DOUBLE INVERSION
It’s a fact that doing a nucleophilic substitution starting with an alcohol would be difficult since the –OH group is a poor leaving group. Fortunately, there are several ways in which the hydroxyl (OH) group can be converted into a good leaving group prior to nucleophilic substitution reaction. One method is to convert an alcohol (R―OH) to an alkyl chloride (R―Cl) via SOCl2 in pyridine or alkyl bromide (R―Br) using PBr3. By doing such reactions, the poor leaving group –OH is converted into a good leaving group in the form of –Cl or –Br.
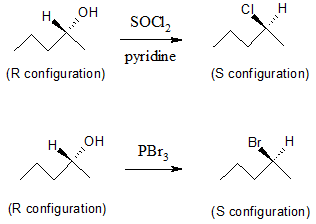
Using SOCl2 or PBr3 in converting alcohols to alkyl halides involves the SN2 mechanism, hence, there is an inversion of configuration as can be seen in the examples above.
Another method for converting alcohols into good leaving groups is by transforming the alcohol into sulfonate ester derivatives such as tosylates (Ts), mesylates (Ms) and triflates (Tf) which are all considered better leaving groups than a OH group. Using this route, the C―O bond in the molecule is not broken which results in a retention of configuration at the stereogenic center.
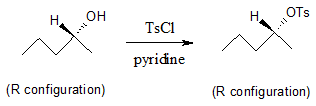
Regardless of which method you choose from above, once the OH group is converted into a good leaving group, a nucleophilic substitution reaction can occur. If the leaving group is on a primary or secondary carbon an SN2 mechanism is favored.
Single Inversion versus Double Inversion
Now let’s turn our attention to the concept of single inversion versus double inversion. Below are three reactions that begin with an alcohol conversion into a good leaving group and then are followed up with an Sn2 reaction by a nucleophile (Nu:-)
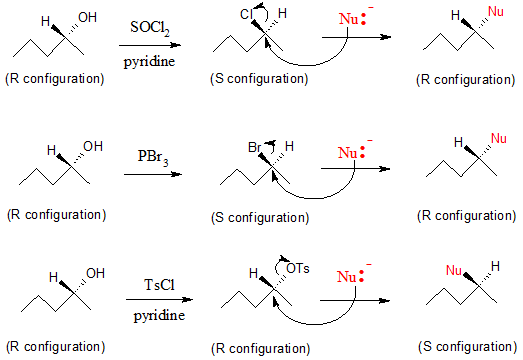
From the first two reactions above, we can see an occurrence of DOUBLE inversion. As mentioned earlier, the use of SOCl2 and PBr3 happens via SN2 mechanism, which results in an inversion of configuration from (R) alcohol to (S) alkyl halide. After the back-side attack by the nucleophile
(Nu:-) for the subsequent reaction, another inversion of configuration occurs which result in an (R) substitution product. We can say that with double inversion, the configuration from the starting alcohol to the final substitution product is retained or the same.
On the contrary, the third reaction involving tosylates illustrates a SINGLE inversion. Inversion of configuration only occurs with the nucleophilic attack to the tosylate product. Hence, in single inversion, there is a net inversion of configuration from an (R) alcohol to an (S) substitution product.
The summary below should help you keep these points in check: