Conversion of Alcohols to Alkenes
Conversion of Alcohols to Alkenes
One way to introduce a π bond into a molecule is to eliminate water from an alcohol in a dehydration reaction. The general reaction is shown below.
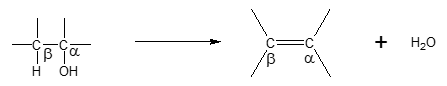
Looking at the reaction above, dehydration is a β elimination reaction in which OH and H are removed from the α and β carbon atoms, respectively. [TR1] For any given alcohol molecule, the α carbon bears the OH group while the β carbon could be any neighboring carbon adjacent to the α carbon. Another important feature to consider for β elimination to occur is that the β carbon must contain an H atom. If the β carbon is bonded to more than one H atom, then any of the H atoms could be eliminated in the process together with the OH group of the α carbon, resulting in the alkene product.
In a mixture of products, there is always a major product, and alcohol dehydration is not an exception. How do we know which alkene is the major product? We’ll talk about that shortly.
Two commonly used reagents in carrying out alcohol dehydration are strong acids such as sulfuric acid (H2SO4) and phosphorus oxychloride (POCl3) in the presence of an amine base. Let’s take a closer look at each of these methods.
Alcohol Dehydration with Strong Acids
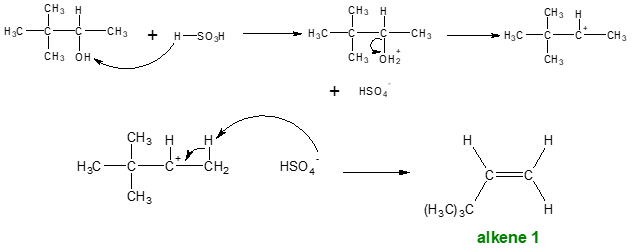
Example:
The degree of alcohol used affects the ease of dehydration, that is, more substituted (higher degree) alcohols dehydrate more readily, giving rise to the order of reactivity below:
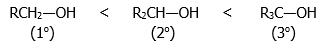
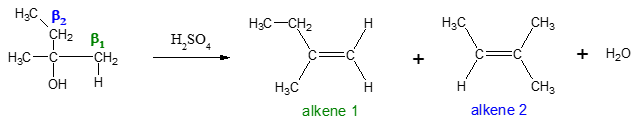
As indicated in the example above, the starting alcohol contains two β carbons (β1 and β2). The loss of H2O from the α and β1 carbons yields alkene 1 while the loss of H2O from the α and β2 carbons produces alkene 2.
Dehydration is regioselective (regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions) and follows Zaitsev rule.
Zaitsev Rule: The more substituted alkene is the major product when a mixture of constitutional isomers are possible. Recall, an alkene with more carbons bonded to the sp2 carbons is more stable.
Thus, alkene 2 would be the major product since it is the more substituted alkene compared to alkene 1.
Aside from sulfuric acid, p-toluenesulfonic acid (TsOH) is also employed for the same purpose.
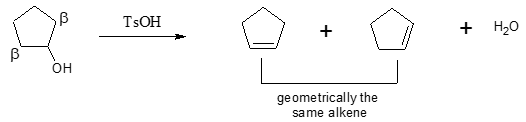
In the above example, although there are two β carbons, they are not distinct to each other, producing geometrically the same alkenes. So, only one alkene is produced in the said dehydration.
It is also important to mention that dehydration using strong acids can occur either E1 or E2 mechanism. As a review, E1 and E2 both begin with the protonation of the OH group, converting it to a good leaving group, but they differ on what happens next. In E1, the loss of the leaving group (H2O in this case) occurs first before the removal of a β hydrogen while in E2, the loss of H2O and removal of the β hydrogen occurs simultaneously. E1 mechanism is commonly observed for 2o and 3o alcohols while 1o alcohols prefer the E2 mechanism. The instability of the carbocation from 1o alcohols forces them to proceed via E2 mechanism.
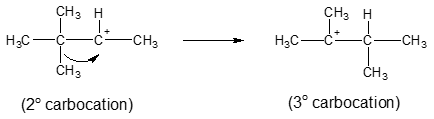
Unexpected results are observed with dehydration occurring via E1 mechanism wherein the double bond is formed in an unexpected location. The reason behind this is that carbocation intermediate in E1 mechanism could rearrange to a more stable carbocation. These possible rearrangements are technically called 1,2-shifts, because they involve migration of an alkyl group or hydrogen atom from one carbon to an adjacent carbon atom. Look at the example that follows for a clearer picture of the said rearrangement.
Normally, we could have the following:
But the secondary carbocation intermediate above could be converted to a more stable tertiary carbocation via 1,2-methyl shift which alters the resulting alkene:
Rearrangement step:
β-hydrogen elimination and alkene formation step:
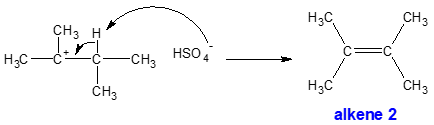
Surprisingly, alkene 1 (monosubstituted) is not observed since the more stable alkene 2 (tetrasubstituted) is preferred.
Dehydration of Alcohols using POCl3 and Pyridine
In some cases, organic compounds in strongly acidic environment could decompose. So how can we allow dehydration of alcohols to occur without the help of either H2SO4 or TsOH? A common option would be the use of phosphorus oxychloride (POCl3) in pyridinePOCl3 converts the poor leaving group like OH into a good leaving group while the presence of pyridine acts as a base and removes a β hydrogen during the elimination. With this reagent, the formation of alkene proceeds via E2 mechanism. The use of this method also gets rid of the complication of rearrangement associated with E1 mechanism. An example is shown below.
Conversion of OH into a good leaving group:
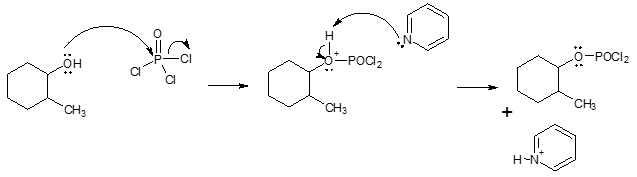
Alkene formation step:
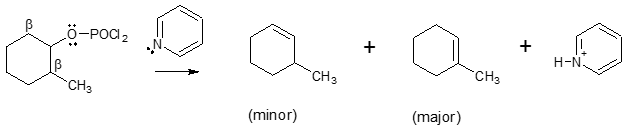
Like in dehydration with strong acids, the use of POCl3 in pyridine also follows Zaitsev rule, making the more substituted alkene as the major product, as indicated in the above reaction.
There you have it, depending on the availability of reagents and specificity required, you can produce alkenes from alcohols using H2SO4 (or TsOH) or via POCl3 in pyridine.
