Alcohols as Acids and Bases
Alcohols as Acids and Bases
Most organic reactions can be simply viewed as acid-base reactions. Reaction of alcohols is not an exception that is why in order to fully grasp the concept of the said reactions, understanding the acidic and basic properties of alcohols would be a good starting point.
Basicity of Alcohols
The hydroxyl group (-OH) gives alcohols many properties that are similar to those of water. Strong acids donate a proton to water as shown in the reaction below.
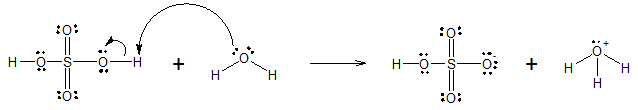
Similarly, in the presence of strong mineral acids, an alcohol also acts as a base. The alcohol accepts the proton giving an alcohol cation called an oxonium ion. The overall general reaction of protonation of alcohols is given below.
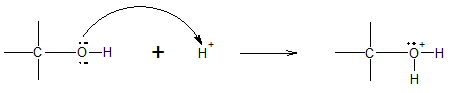
The above reaction clearly shows that the basic property of alcohols utilizes the Lewis definition of bases, that is, as electron pair donors.
Acidity of Alcohols
An alcohol can also act as an acid and donate proton to a base which is stronger than the alkoxide ion (RO-), the conjugate base of the alcohol. This behavior utilizes the concept of Bronsted-Lowry definition of an acid which states that an acid is a substance that donates proton (H+).
Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Thus, sodium hydroxide (NaOH) is not a strong enough base to generate a significant concentration of alkoxide ion from alcohol, that is, at the observational level, alcohols do not react with sodium hydroxide.
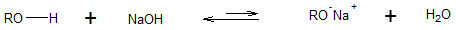
Notice that the above reaction is a reversible one, and the double arrow indicates that the backward reaction is more favored compared to the forward reaction. This supports the fact that alcohols don’t readily react with hydroxides (OH-).
If you wish to take advantage of the acidity of alcohols and produce alkoxide ions, reacting alcohols with metal hydrides (H:-) proceeds to completion producing hydrogen gas (H2) along with the alkoxide.
Example:
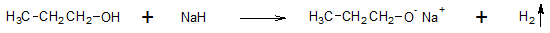
Like water, alcohols react vigorously with active metals such as sodium or potassium to evolve hydrogen gas.
Example:

Factors Affecting the Acid Strength of Alcohols
Just like any other organic compounds, similar factors affect the acidity of alcohols such as resonance effect and inductive effect. The bottom line when comparing acid strengths of different alcohols is to consider how the negative charge the resulting alkoxide ion is stabilized after the alcohol is deprotonated.
How is the negative charge stabilized in a given alcohol molecule? The idea is that we want the negative charge to be spread over the molecule and one way for electron density to be distributed over the entire molecule is through resonance. This is main reason why resonance effect has a big influence on the acid/base properties of organic compounds including alcohols.
It would be an advantage if one can able to compare acid strengths of compounds based on their structure rather than simply comparing pKa values.
Factor 1: Resonance Effect
The point is that alcohols where the corresponding alkoxide ion is resonance stabilized will be more acidic than those without resonance. A very good example to illustrate the effect of resonance on the stability of the alkoxide anion is to compare the acidity of phenol and cyclohexanol.
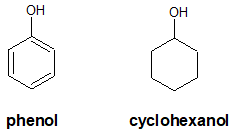
Let’s consider first the alkoxide anion formed from cyclohexanol.
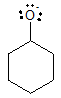
In the above structure, resonance would not be possible. As a result, the negative charge is concentrated only on the oxygen atom.
Now, let’s look at phenoxide ion, the conjugate base of phenol.
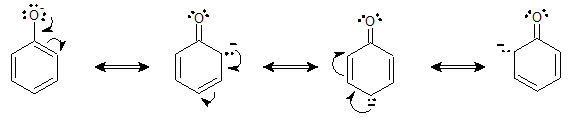
It is evident that the phenoxide ion has three possible resonance structures, showing that the negative charge is well distributed throughout the entire molecule. Hence, phenol is more acidic than cyclohexanol for the reason that the conjugate base of phenol is more stable (resonance stabilized) than that of cyclohexanol.
Factor 2: Inductive Effect
Inductive effect is an electronic effect due to the distortion of electron density of a single () bond within a molecule or ion. It is typically due to an electronegativity difference between the atoms at either end of the bond. The more electronegative atom pulls the electrons in the bond towards itself creating some bond polarity. Let’s look at ethanol and trichloroethanol to illustrate inductive effect.
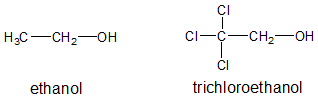
The conjugate base of ethanol and trichloroethanol are also shown below:
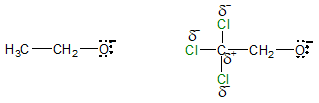
Notice that for the anion from ethanol, the negative is distributed mostly to the oxygen atom. On the other hand, the presence of electronegative chlorine (Cl) atoms on the adjacent carbon makes the carbon very electron deficient which in turn reduces the negative charge on the O atom. This accounts for the greater acidity of trichloroethanol compared to ethanol, that is, inductive effect present in trichloroethanol reduces the negative charge on the oxygen atom while there is none on ethanol.
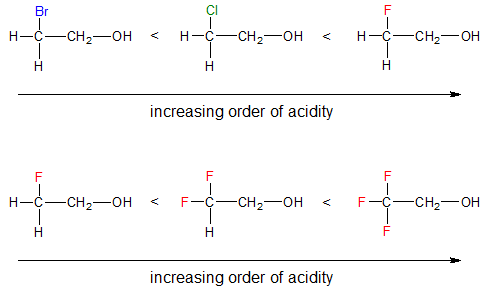
Take note that the higher the electronegativity of the atoms attached to the alcohol, the more acidic the alcohol becomes. Mostly halogens (F > Cl > Br > I) causes inductive effect and down a group, the enhanced acidity due to inductive effect decreases due to the decrease in electronegativity. The greater the number of electronegative atoms present in the molecule, the greater would be its acidity due to increased stabilization of the corresponding conjugate base or alkoxide ion.