Pinacol Rearrangement
Pinacol Rearrangement
Generally speaking, organic reactions are believed to proceed with minimal structural change which makes it easier for us to predict and elucidate the products of any substitution, addition or elimination reaction. This notion also enables one to characterize the chemical behavior of common functional groups present in organic compounds. But in a sense, there is always what we call “deviation from the norm”.
We’re talking about the case wherein the product of a given organic reaction is structurally not as we expected it to be. Or at least not at first glance. These reactions usually involve cationic intermediates and in the case of carbon, are carbocations. As a review, a carbocation is a molecule in which a carbon atom bears three bonds and a positive charge.
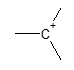
In general, the structural change occurring in carbocations is mainly because they tend to rearrange to a more stable carbocation via hydride or alkyl shift. We will describe and discuss one of the intriguing reactions involving an alcohol starting material, the Pinacol rearrangement.
Pinacol rearrangement was the first observed molecular rearrangement. To start with, let’s take a look at the mechanism involved.
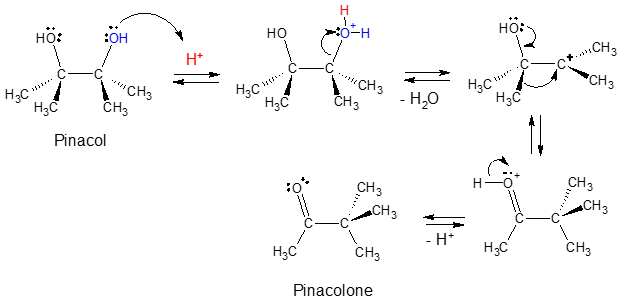
Notice that since the diol structure of pinacol is symmetrical, protonation and loss of water can take place in either of the OH group. It is interesting to mention that after the loss of water, a stable tertiary (3o) carbocation is formed as the initial intermediate.
Normally, a rearrangement is observed when a less stable carbocation (like 2o carbocation) is converted to a more stable form (like 3o carbocation) via 1,2-hyride or methyl shift. But in the case of pinacol, we observe a rearrangement from a tertiary cation to a secondary! So, what then drives the rearrangement to occur even when the initial intermediate is already a tertiary carbocation? The answer to this question lies in the additional stabilization caused by charge delocalization as a result of heteroatom resonance. This event is highlighted in the figure below.
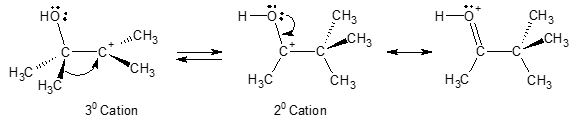
Recall that resonance is one of the factors that could be considered to account for the enhanced stability of a given organic compound. With the 1,2-methyl shift shown in the mechanism of pinacol rearrangement to pinacolone, an even more stable carbocation in which the charge is delocalized by resonance is generated.
