Oxidation of Alcohols
OXIDATION OF ALCOHOLS
One of the most valuable reactions of alcohols is their oxidation to form carbonyl compounds such as aldehydes, ketones and carboxylic acids. The outcome of the oxidation reaction of alcohols depends on the degree of substitution of the carbinol carbon (the term used to refer to the carbon bearing the OH group). Let’s take a look at each degree of alcohols and their oxidation.
Oxidation of Primary (1o) Alcohols
Primary alcohols can be oxidized to aldehydes or further to carboxylic acids.
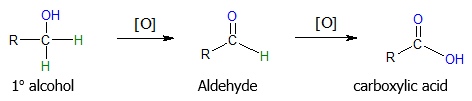
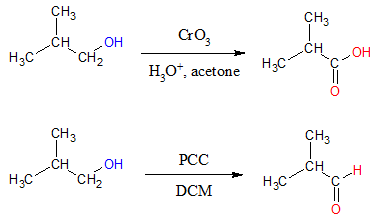
The result of the oxidation of primary alcohols depends on the reagent used to carry out the reaction. If you want to convert primary alcohols directly to carboxylic acids, strong oxidizing agents such as KMnO4, CrO3 and Na2Cr2O7 are employed. In the case when you want the oxidation to only yield aldehydes, one of the best methods would be using pyridinium chlorochromate (PCC) in dichloromethane (DCM) solvent. Examples are shown below.
Oxidation of Secondary (2o) Alcohols
In the case of secondary alcohols, their oxidation reactions give high yields of ketones.
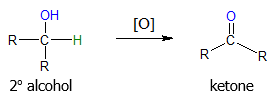
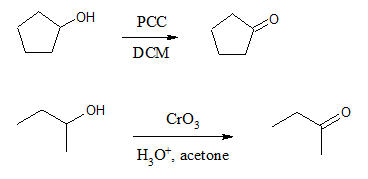
Any of the reagents mentioned in the oxidation of primary alcohols can also be used to carry out the oxidation of 2o alcohols to ketones. Both strong and mild reagents result to a ketone product unlike the case of primary alcohols wherein milder reagents yield aldehydes while stronger oxidizing agents produce carboxylic acid. Specific examples are illustrated below.
Oxidation of Tertiary (3o) Alcohols
Tertiary alcohols do not react with most oxidizing agents. Hence, oxidation of 3o alcohols is not observed.
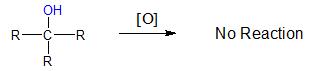
Why are tertiary alcohols not oxidized unlike primary and secondary alcohols? Aside from the presence of an oxidizing agent, a hydrogen atom attached to the carbinol carbon is also important. If you recall the general structures of 1o, 2o and 3o alcohols, notice that primary and secondary alcohols have an H atom still bonded to the carbon bearing the OH group while tertiary alcohols have no available H atom at the carbinol carbon. This is the main reason why tertiary alcohols do not undergo oxidation.
All oxidation reactions of primary and secondary alcohols are closely related to an E2 reaction.
Mechanism:
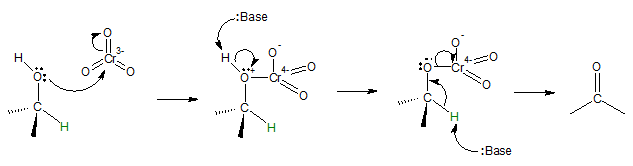
The green hydrogen bonded to the carbinol carbon in the mechanism illustrated is lost as H+ by abstraction of a base and the pair of electrons in the C-H bond completes the formation of C=O, resulting to the oxidation of C-OH bond in the alcohol compound. Recall that you can view oxidation in a number of different ways such as loss of electrons or addition/removal of certain atoms. In the case of alcohols, oxidation occurs when H atom(s) from the starting compound is(are) lost or O atom(s) is(are) added. For tertiary alcohols, there is no H atom bonded to the carbinol carbon that can be abstracted by the base and the C=O formation is inhibited.
