Oxidative Cleavage of Diols
OXIDATIVE CLEAVAGE OF 1,2-DIOLS
Oxidative cleavage reactions are mostly encountered in compounds containing carbon-carbon double bonds or simply called alkenes. One of the purposes for carrying out this reaction is to determine the the position of a double bond in an alkene compound. The most common method used is ozonolysis, using O3 to cleave the double bond, resulting to aldehydes and ketones. See an example below.
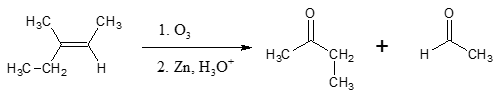
Interestingly, we can do the same reaction as above using another method, one which involves a di-alcohol compound known as a vicinal diol. This article focuses mainly on this second method.
Conversion of Alkenes to Vicinal Diols (or Glycols)
Before we go to the cleavage reaction part, let’s first talk about the formation of vicinal diols. Dihydroxylation (addition of two (2) OH groups) of alkenes can be accomplished via two methods. One method involves the use of peroxyacids such as meta-chloroperoxyacetic acid (mCPBA) to form an epoxide ring in the carbon-carbon double bond followed by treatment with aqueous acid.
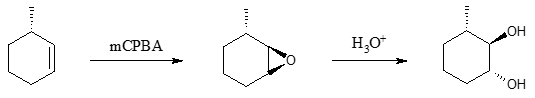
Notice that the addition of acid causes the epoxide ring to open, which leads to a diol product. Take note also that the above reaction yields a specific trans isomer of the diol.
The second method in generating vicinal diols from an alkene starting material is the utilization of osmium tertroxide (OsO4). The method yields a cis diol product.
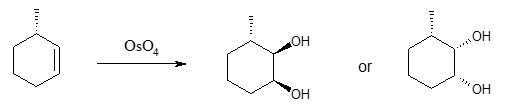
The drawback of using OsO4 is that it’s quite expensive. But depending on the stereochemical requirement, you can choose between the two methods. As a summary, epoxidation followed by acid treatment of alkenes results to trans diols while the use of OsO4 yields cis diols. Both methods produce a vicinal diol. What is meant by the term vicinal? Like what were illustrated in the examples, vicinal diols are compounds containing adjacent OH groups. Sometimes it is also called 1,2-diols, indicating that the two (2) OH groups, regardless of the carbon skeleton size of the compound, are always next to each other.[TR1]
Oxidative Cleavage of Vicinal Diols
Just like in ozonolyis, the starting diol compound is cleaved, producing two new carbonyl compounds. It could be an aldehyde or a ketone, depending on the substitution involved in each carbon bearing the OH group. Unlike ozonolysis where the C=C is cleaved directly, the method involving vicinal diols occurs with the cleavage of C―C step.
How is the cleaving carried out? There are two reagents that you can choose from: (1) periodic acid (HIO4) in H2O and THF as solvent or (2) lead tetraacetate (Pb(OAc)4) in benzene or acetic acid. The mechanism of both reactions is believed to occur with a heterocyclic intermediate, as shown in the mechanism that follows.

We can summarize the mechanism as a rearrangement of electrons in the intermediate, cleaving a C―C bond and formation of two (2) C=O. As you can see, either reagent will produce the same final product. In this case, the starting 1,2-diol is cyclic, so the cleavage of the C―C bearing the OH groups results to single molecule containing to carbonyl groups at each end. In the case of non-cyclic diol compounds, two separate carbonyl compounds are observed after oxidative cleavage.
Non-cyclic example:
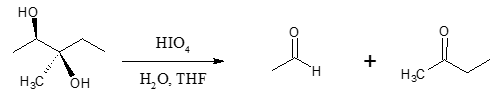
When can we get an aldehyde or a ketone as a product? Another important aspect to consider is the type of carbonyl compound formed after the cleavage. In the example above, one of the carbons bearing the OH group is considered secondary (2o) since it is bonded to two other carbon groups. If this is the case, the resulting carbonyl compound is an aldehyde. This suggests that when the carbon in the C―C bearing the adjacent OH groups is a tertiary (3o) carbon center, the cleavage results to a ketone product.
Last point about oxidative cleavage of vicinal diols that is worth mentioning is the comparison of rate of reactivity between cis and trans vicinal diols. From the given mechanism, it is easy to see that cis diols react faster as compared to trans diols. This difference in reactivity could be attributed to orientation factor which affects the formation of the heterocyclic intermediate.
What is the advantage of oxidative cleavage of vicinal diols over ozonolysis with respect to oxidative cleavage of alkene in general? Oxidative cleavage of alkenes via vicinal diol pathway results to higher yields compared to ozonolysis route. In fact, the former method is often preferred when it comes to small scale work involving valuable compounds.
