Nomenclature of Alcohols
Nomenclature of Alcohols
Alcohols occur widely in nature and have many industrial and pharmaceutical applications. For instance, ethanol, is known to be produced from fermentation of grains and sugars in wine making and for biofuel production.
Alcohols are organic compounds with characteristic hydroxyl (―OH) functional group attached to a saturated, sp3-hybridized carbon atom.
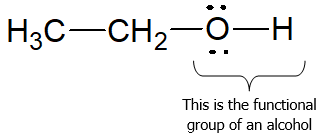
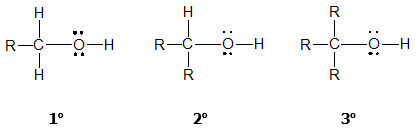
The structure of alcohols can be viewed in two ways: (1) as hydroxyl derivatives of alkanes; and (2) as alkyl derivatives of water. They are classified as primary (1o), secondary (2o) or tertiary (3o) based on the number of carbon groups (R) bonded to the carbon with the OH group.
To name an alcohol using the IUPAC system, we must learn how to name the functional group by using a suffix added to the parent alkane. This means that if you know already the basic rules in naming hydrocarbons such as alkanes, then the only adjustment is using the suffix –ol.
Consider the organic structure below and follow the steps in naming alcohols according to IUPAC rules:
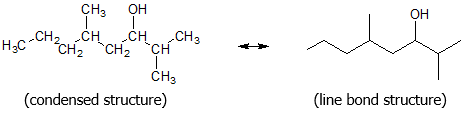
Step 1: Select the longest carbon chain containing the carbon bonded to the OH group.
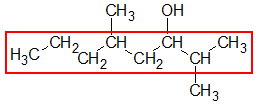
Looking at the given structure, the longest carbon chain would be the one indicated by the red box. Obviously, the parent chain contains 8 C’s. Give the name of the parent alkane by using the appropriate prefix indicating the number of carbons and change the –e ending to the suffix –ol.
8 C’s → octane → octanol
Hence, the parent name of the given compound is octanol.
Step 2: Number the parent chain in such a way that the carbon bearing the OH group has the lowest number possible. It is important to note that you always number from an end of the carbon chain. You cannot number the carbon bearing the OH position 1 unless it is an end carbon. Your goal is always to start numbering the end closest to the carbon bearing the OH group. This is necessary since the position of the OH functional group in the given compound must be indicated in the name.
There are two possible numbering sequences:
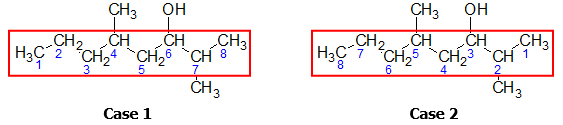
Comparing the two numbering cases, Case 2 should be followed since the OH group is found at C-3 rather in C-6 in Case 1. The number of the carbon atom containing the OH group must then be incorporated in the parent name to emphasize the position of the hydroxyl group.
At this point, the parent name would then be 3-octanol. (Note: Always use dash (-) when separating numbers and letters)
Step 3: After completely identifying the details of the parent name, apply all other general rules for nomenclature such as adding the name of the substituents in the given structure. Don’t forget to indicate the position of the carbon at which these substituents are attached.
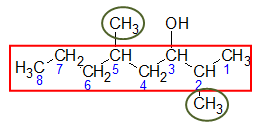
In this case, the substituents are two -CH3 groups (encircled in green) at carbons 2 and 5, respectively. Recall that a substituent with 1 carbon is named methyl and if the same substituent occurs in the structure more than once, the name of the substituent is mentioned with the appropriate prefix indicating the frequency of appearance in the structure.
Since substituents come first before the parent name, the complete name of the given alcohol would then be:
2,5-dimethyl-3-octanol
There is a variation of the above name, wherein the position of the carbon bearing the OH group is placed next to the suffix. The name then becomes:
2,5-dimethyloctan-3-ol
Both conventions are valid names of the given compound. It depends now on which convention your instructor or professor prefers to use in class. It would be ideal to just follow the convention used during your lectures.
What if the structure in consideration contains more than one OH group? How do we properly give the name then? Consider the next structure below.
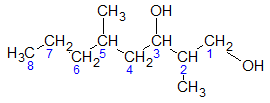
The above structure is similar to the first example except that there is an additional OH group attached to C-1. Can you see it?
Now, the treatment of two (2) or more of the same functional groups is the same as having two or more substituents. What I mean is that you just have to indicate the positions of the carbons bearing the said OH groups and incorporate them in the parent name. In addition, don’t forget to use the appropriate prefix representing the number of occurrence of the OH group.
Going back to the second structure, it has 2 OH groups which tells us that the prefix di- must be used. Hence, the name would 2,5-dimethyl-1,3-octanediol. Notice that the –e ending of the name of the parent alkane is retained in this case.
For cyclic compounds, recall that the prefix cyclo- is used to emphasize that the compound is in cyclic form. As with aliphatic hydrocarbons, just change the ending –e to suffix –ol. The numbering of the carbon atoms in the ring is easier for cyclic compounds since the carbon bearing the OH group is always assigned as C-1, provided that –OH is the only functional group present in the structure and the substituents are just alkyl groups (-R) or halogens (-X). An example is given below.
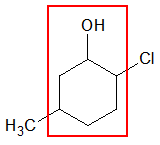
The parent cycloalkane in the above structure (represented by the red box) is a 6-membered ring named as cyclohexane. Since it contains an –OH group, the name is adjusted as cyclohexanol.
To properly indicate the positions of the substituents, you must number the ring clockwise starting at the carbon bearing the OH group. Numbering the ring clockwise will give a smaller set of values for the positions of the substituents rather than going counter-clockwise.
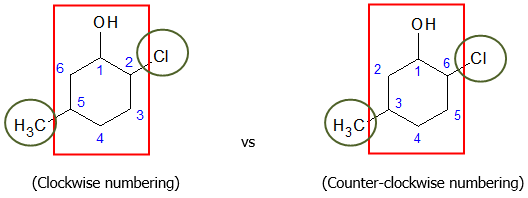
Hence, the complete name of the given cyclic compound would be
2-chloro-5-methylcyclohexanol
Notice that there is no need to indicate the position of the OH group for cyclic compounds since is it understood that the carbon bearing the OH group is C-1.
