Physical Properties of Alcohols
PHYSICAL PROPERTIES OF ALCOHOLS
Alcohols are one of the most important molecules in organic chemistry. They contain the hydroxyl functional group (-OH), bonded to a carbon atom of a hydrocarbon chain of varying lengths. The two covalent bonds, C–O and O–H bonds, of the compound influence the intermolecular forces of attraction (IMFA) present in alcohols. If you recall, the type of IMFA present in a given molecule relates to the different observed physical properties. And in this article, we will visit the following properties of alcohols: (i) structure and polarity, (ii) boiling point, (iii) solubility, and (iv) viscosity.
Structure and Polarity of Alcohols
Alcohols contain an oxygen atom surrounded by two atoms and two lone pairs, making the O atom of the hydroxyl group tetrahedral and sp3 hybridized. The electron domains previously mentioned also give rise to the bent shape of alcohols just like water (H2O).
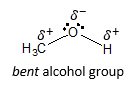
Since O atom is much more electronegative than C or H atom, the C–O and O–H bonds are considered as polar, with the electron rich O atom and the electron poor C and H atoms. This is designated by the partial positive and partial negative charge illustrated in the above structure. Clearly, alcohols have a degree of molecular polarity (not just by virtue of polar bonds but being polar at its entirety as a molecule) because there is always a net dipole moment present in a bent molecule. This means that the polarity of the bonds will not cancel out regardless of the orientation of the dipole moment of the polar bonds surrounding the O atom in the hydroxyl group.
Intermolecular Forces of Attraction in Alcohols
Now that we’ve established that the presence of -OH group in alcohols gives them certain degree of polarity, let’s take a closer look at how alcohol molecules interact with their neighbouring alcohol molecules. I’m talking about the intermolecular forces of attraction present in alcohols. Before we go to the different physical properties, it is important to understand first the forces that give rise to such properties.
Being a polar molecule, we can say that dipole-dipole interaction is present between alcohol molecules. In fact, a special case of such interaction which we call hydrogen bonding is the predominant IMFA in most alcohols. As a review, hydrogen bonding occurs between molecules wherein an H atom is attached to a highly electronegative atoms namely fluorine (F), nitrogen (N) or oxygen (O). Obviously, H is bonded to an O atom in the hydroxyl group which makes alcohols capable of hydrogen bonding as illustrated by the red dashed bond in the figure that follows.
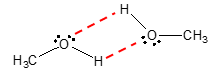
The presence of hydrogen bonding in alcohols explains why they have relatively higher boiling points that their alkane counterparts.
Another equally important IMFA present in alcohols are the London dispersion forces (or van der Waals forces). The said IMFA is commonly encountered in nonpolar molecules such as alkanes. Alcohols are considered as derivatized alkanes in a sense that the hydrocarbon chain is only bonded to an OH group. Hence, London dispersion is also present in alcohols along with hydrogen bonding.
The two intermolecular forces mentioned will help us understand the trends in boiling points and solubilities of the different alcohols which will be discussed shortly. What I’m trying to say is that the patterns in the different physical properties reflect the patterns in intermolecular attractions.
Boiling points of Alcohols
First of all, alcohols have relatively higher boiling points than their alkane counterpart due to the reason that only London dispersion is present in alkanes which is a weaker intermolecular force than hydrogen bonding that is predominantly present in alcohols. What I’m saying is that the stronger the strength of IMFA present, the higher would be the value representing the property of the molecule. Let’s now look at the trend in boiling points of different alcohols.
Shown below are the boiling points of some simple primary alcohols up to 5 carbons:
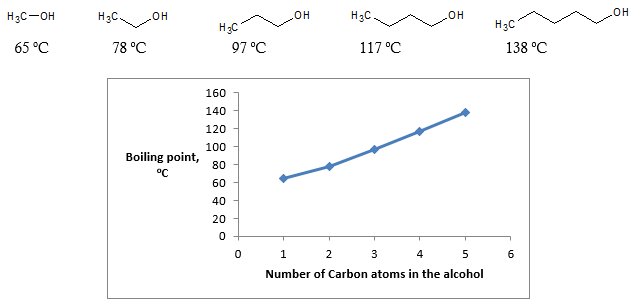
As you can see, as the number of carbons in the alcohol increases, the boiling point also increases. If you look at the structure of the alcohols compared, all of them contain one OH group which means that hydrogen bonding would be relatively the same throughout the series of alcohols. What then causes the difference in boiling points? This is where you’ll appreciate the effect of van der Waals forces (or London dispersion). There is an increase in strength of London dispersion as the length of hydrocarbon chain increases. So aside from hydrogen bonding, the effect of van der Waals forces also contributes to the observed boiling point of the alcohol especially as the hydrocarbon chain becomes longer.
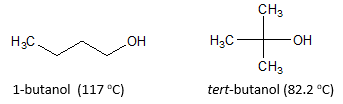
Let’s look at a different scenario wherein we compare the different isomers of alcohol. As an example, refer to the figure above about 1-butanol and tert-butanol. Both alcohols have the same number of carbons but they have different boiling point values. How can we account for this difference in boiling points? The factor that we need to consider is the surface area of the molecule. As expected, straight chain molecules will have higher surface area than their branched counterpart. It is good to note that van der Waals interaction in the hydrocarbon chain of alcohol or in general increases as the surface area increases. Hence, the straight chain 1-butanol has a higher boiling point (117 oC) compared to the branched tert-butanol with boiling point of only 82.2 oC.
Note that similar trend as in boiling point is observed when we consider melting points of alcohols.
Solubility in Water
When we talk about solubility, you may recall the golden rule “Like dissolves Like” which simply means that polar substances are soluble with other polar substance and nonpolar substances are soluble in other nonpolar substances. Using the same argument, alcohols are soluble in water since both are polar molecules. But as the length of hydrocarbon chain in the alcohol increases, there is an appreciable decrease in solubility in water. Since alcohols are liquid and water is also liquid, the right term that must be used to describe the solubility of alcohols in water would be “miscible”. As can be seen in the table below, as the number of carbon increases, the alcohol becomes less miscible in water.

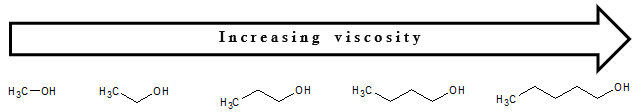
Viscosity
As a review, viscosity is the property of a fluid that resists the force tending to cause the fluid to flow or simply put it as the resistance of a liquid to flow. If we consider alcohols, their viscosity increases as the size of the molecules increases. That means as the hydrocarbon chain of the alcohol increases, the higher would be the viscosity. You can account this observation still due to the increased intermolecular force of attraction as the number of carbon atoms in the alcohol increases.