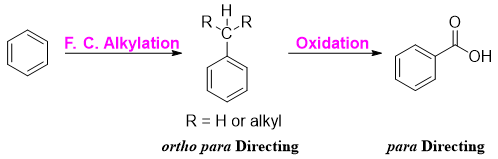
Side chain Oxidation
Side-chain Oxidation
Oxidation reaction that takes place at a group directly attached to a benzene ring is termed as side-chain oxidation. In this reaction, alkylbenzenes are readily oxidized by alkaline KMnO4 or acidic K2Cr2O7 or dilute HNO3. In these kinds of reactions, the alkyl groups are oxidized keeping the benzene ring intact.
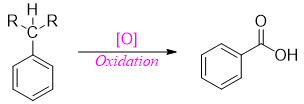
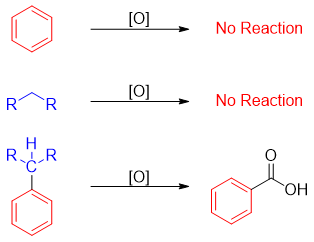
Side-chain oxidation of alkylbenzene takes place when there is at least one benzylic hydrogen attached to the carbon. No reaction occurs in the absence of benzylic hydrogen. Thus, these reactions have no effect on tertiary carbons attached to an aromatic ring.
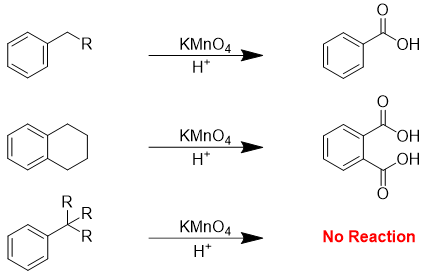
Whatever the length of an alkyl group may be, it gives only on carboxyl group as a product after oxidation reaction. Following example demonstrates the utilization of oxidation reaction in the production of substituted benzoic acid.
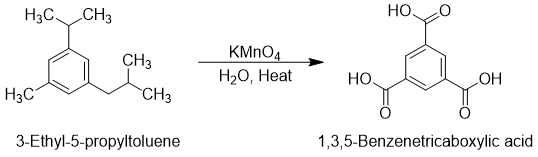
Both alkane and benzene are very stable and insusceptible to oxidation but when put together, the benzylic carbon which is directly attached to the benzene ring becomes more reactive and more susceptible to oxidation.
The mechanism of oxidation reaction occurs at benzylic position is not well known. It is thought that the first step involves the removal of the hydrogen atom by oxygen atom of MnO4- via free radical reaction.
Oxidation of alkylbenzene containing benzylic protons by KMnO4 is used as identification test. KMnO4 solution having purple color decolorizes once consumed in the oxidation reaction. Therefore, one can easily differentiate between benzene, benzene containing primary, secondary, and tertiary alkyl groups by chemical test with KMnO4 solution.
Applications of oxidation of alkylbenzene (having benzylic protons) includes the synthesis of benzoic acid and converting ortho and para directing groups into meta directing group.