Halogenation
Halogenation of Benzene
Halogenation of benzene refers to the electrophilic aromatic substitution reactions in which one of the hydrogen atoms on benzene ring is replaced by a halogen atom.
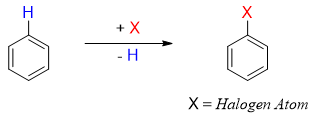
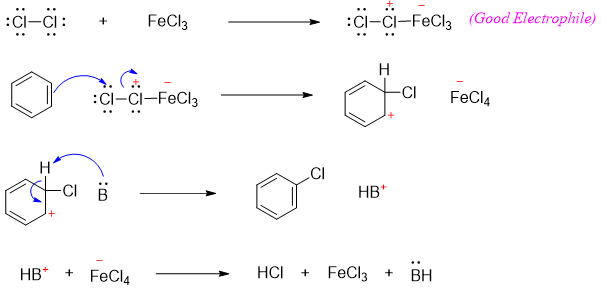
Halogenation reaction of benzene ring is conducted in the presence of Lewis acids. Bromination and chlorination of benzene is performed in the presence of iron bromide and iron chloride.
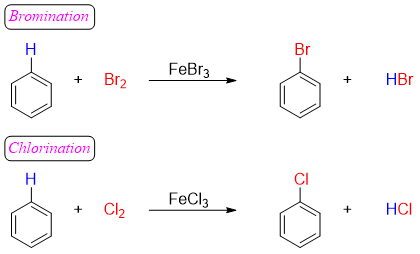
The use of the Lewis acid as a catalyst is very important to generate a strong electrophile. Benzene being more stable does not reacts with weak electrophiles thus, Lewis acids promotes the synthesis of strong electrophile as depicted in mechanism of bromination reaction below.
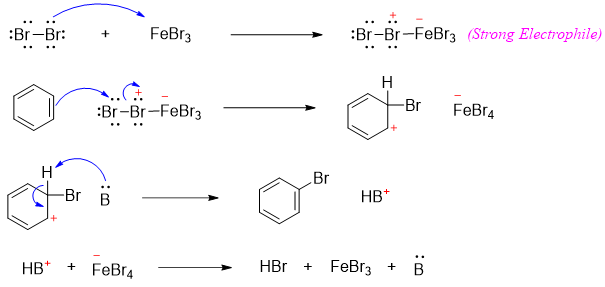
The mechanism of chlorination reaction of benzene is shown below.
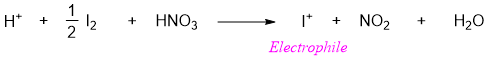
Iodination of benzene ring is done by reacting benzene with I2 in the presence of acidic oxidizing agent i.e., HNO3. The nitric acid used in this reaction does not act as a catalyst instead it is consumed and act as oxidizing agent.
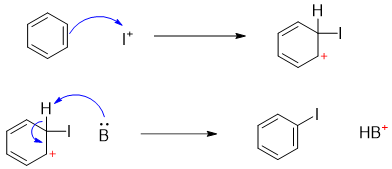
The mechanism of Iodination reaction also involves the production of strong electrophile I+.
The iodine electrophile is added to the benzene ring followed by removal of proton by base to regenerate the aromatic ring.
The direct fluorination reaction of benzene is not possible as the reaction is reversible. The byproduct obtained in direct fluorination reaction of benzene is hydrofluoric acid which is a strong reducing agent thus, it reduces fluorobenzene back to benzene.

As the reaction of benzene with F2 is too reactive and highly exothermic therefore, alternate fluorinating agents are used to fluorinate benzene ring directly. One of the fluorinating agent used is called F-TEDA-BF4 [1-(chloromethyl)-4-fluoro-1,4-diazoniabicyclo [2.2.2]octane ditetrafluoroborate] sold under the commercial name Sectfluor®.
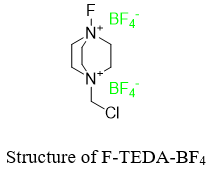
The reaction of benzene with F-TEDA-BF4 is given below.
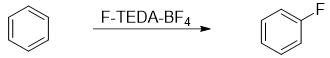
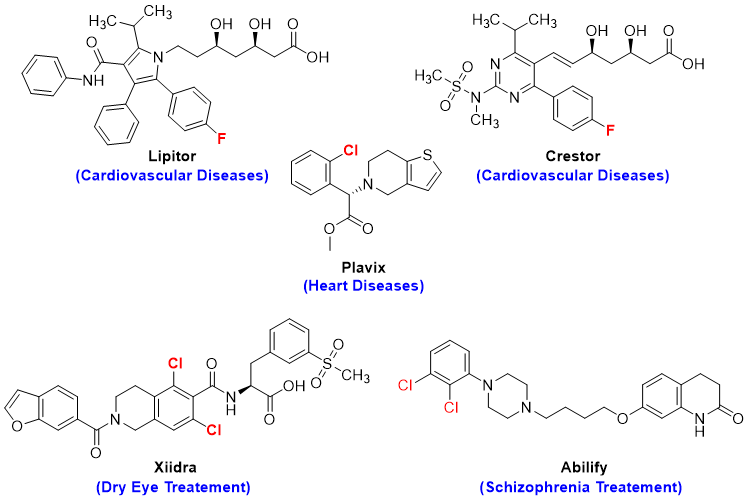
The process of halogenation of aromatic compounds has many applications. Following are some blockbuster drugs used to treat many diseases.