Reduction of Benzyl Carbonyl
Reduction of benzyl carbonyl
In organic chemistry reduction reactions are those reactions in which increase in hydrogen content and decrease in oxygen content take place. The reduction of benzyl carbonyl compound is shown below.
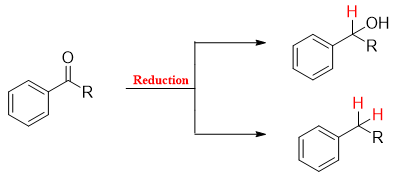
The reduction of benzyl carbonyl compounds is carried out by many different methods. Some of the most common methods are listed below.
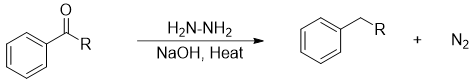
Wolf-Kishner Reduction
This reaction was discovered in 1912. In this method carbonyl group (C=O) is reduced to methylene group (-CH2-). This method not only reduces benzyl carbonyl compounds but also all ketones.
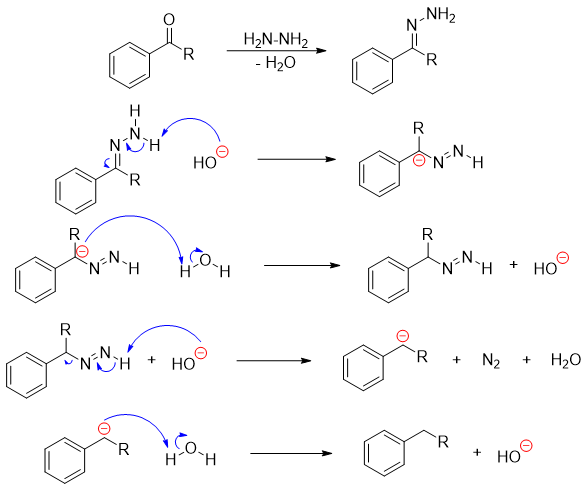
The mechanism of Wolf-Kishner reduction is shown below.
Clemmensen Reduction
In 1913 Densish chemist Clemmensen reported the reduction of ketones and aldehydes to corresponding alkanes using zinc amalgam catalyst using acidic conditions.
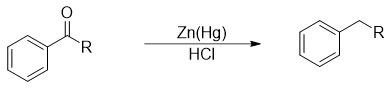
The mechanism of this reaction involves the reduction taking place at zinc metal surface.
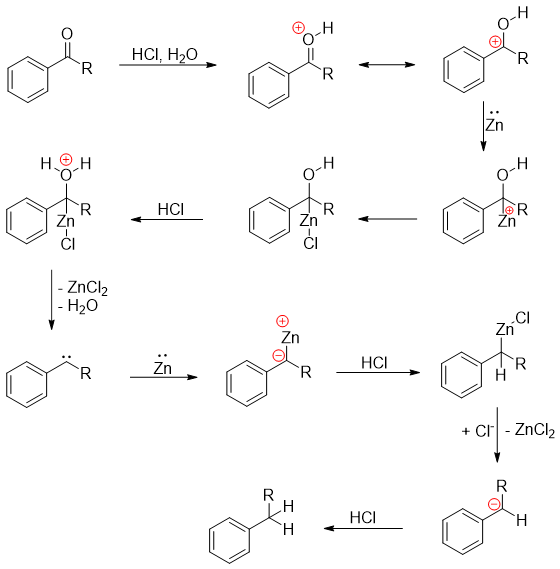
Clemmenson reduction and Wolf-Kishner reduction reactions differ in many aspects. Clemmenson reduction is used to reduce ketones and aldehydes into corresponding alkanes while, Wolf-Kishner reduction is employed to reduce carbonyl functional group into methylene group. Secondly, Clemmensen reduction is carried out in acidic conditions therefore, those substrates which are acidic sensitive can not be reduced using this method. Meanwhile, Wolf-Kishner reduction is carried out in basic medium.
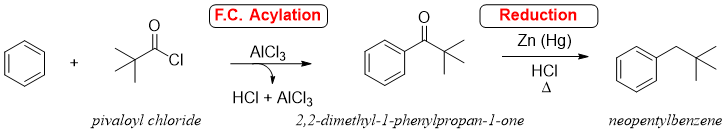
One of the major applications of reduction reaction of benzyl carbonyl compounds is the synthesis of unbranched alkyl benzenes. For this purpose, F. C. acylation is followed by reduction reactions which helps in avoiding the rearrangements of carbocations produced in F. C. Alkylation reactions. The example below shows the synthesis of neopentylbenzene as a sole product via F. C. acylation followed by reduction reaction. This product can not be synthesized directly from F. C. Alkylation reaction.
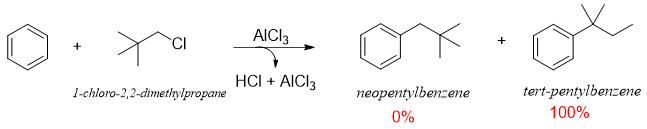
In above reaction neopentylbenzene (desired product) is not formed due to rearrangement of carbocation.
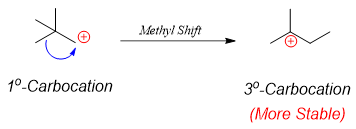
Therefore, F. C. acylation followed by reduction can yield the desired product.