Aromatic, Antiaromatic and Non Aromatic
Aromatic, Antiaromatic and Nonaromatic Compounds
Historically “aromaticity” is a major chemical concept which commenced with the isolation of benzene in 1825 by Michael Faraday. There were many criteria and definitions developed for the identification of aromaticity. Following are some milestones.
- Unique Aromatic smell (before 1825)
- High C:H ratios (before 1865)
- Benzene structure by Kekulé (1865)
- Substitution reactions are more favorable by Erlenmeyer (1866)
- Hückel's rule (1931)
- Ring current theory by Pauling (1936)
- Ring current effects using NMR by Pople (1956)
- Anisotropy Effect by Flygare (1970)
Following factors have converged to following standards of aromaticity.
- Electrophilic Aromatic Substitution reactions
- Cyclic delocalization resulting in equal bond lengths
- Greater stability
- Ring current effects
Aromatic Compounds:
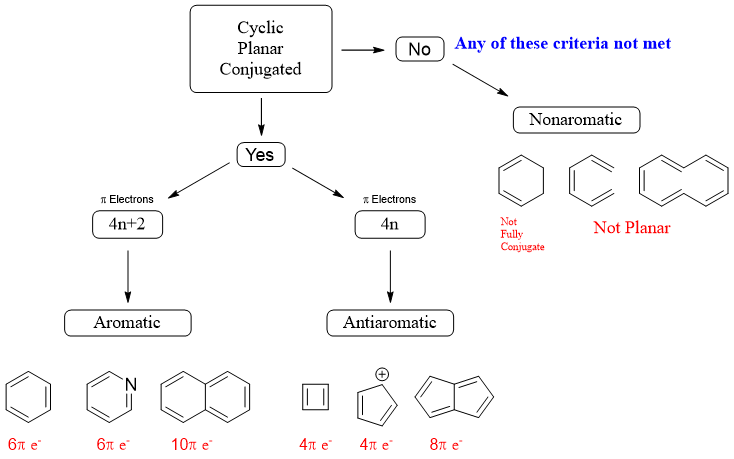
Those compounds which follow following criteria are said to be aromatic compounds.
- The compound must be cyclic
- Every carbon atom of the molecule must have unhybridized p orbital
- The unhybridized p orbital must overlap on either side
- The molecule must be flat
- It must follow Hückel's (4n+2) rule, where n is a whole number
The Hückel's rule is a mathematical way of stating an aromatic compound to have an odd number of pairs of pi electrons.
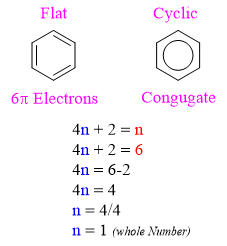
The simple and common example of aromatic compound is benzene. The benzene structure is flat. It has 6 π electrons thus, it follows Hückel's rule. All the carbon atoms have unhybridized p orbitals and all p orbitals are overlapping.
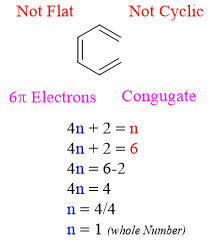
On the other hand, 1,3,5-Hexatriene is not as stable as benzene. 1,3,5-Hexatriene contains 6 π electrons but it does not follow other aromaticity rules like flatness and cyclic ring.
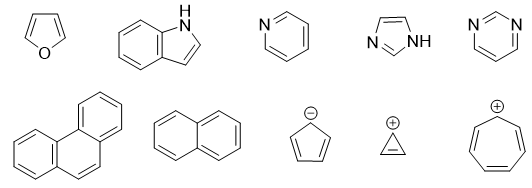
Other examples of Aromatic compounds are shown below.
Antiaromatic Compounds:
Antiaromatic compounds are unusually unstable as compared to aromatic compounds. Following factors determine the molecule to be antiaromatic.
- They are cyclic molecules
- They are conjugated molecules
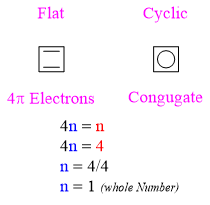
- They contain (4n) π-electrons
- They are flat
Cyclobutadiene is a common example of antiaromatic compounds.
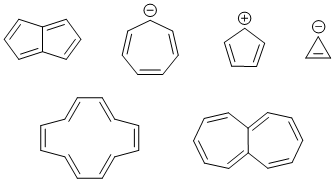
Other examples of Antiaromatic compounds are shown below.
Nonaromatic Compounds:
These are the third class of organic compounds. They neither follow aromatic rules nor antiaromatic rules. Any compound which is not planar, cyclic, and fully conjugated is said to be nonaromatic.
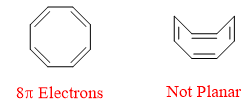
The structure of Cyclooctatetraene is shown below. It is cyclic, follows 4n rule and is fully conjugated. This molecule is nonaromatic in nature because of the fact that this molecule is not flat which is major requirement for a molecule to be aromatic or antiaromatic. The structure of cyclooctatetraene is shown below.
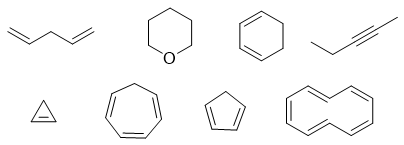
Other examples of Nonaromatic compounds are shown below.
Following chart can be used to classify aromatic, antiaromatic and nonaromatic compounds.