Diazonium Formation
Diazonium Formation
When primary amines are reacted with nitrous acid (HNO2) in the presence of acid produces diazonium salts. This type of reaction is called as diazotization reaction. Amines can be primary arylamines or primary alkylamines. The arenediazonium salts are stable than the alkanediazonium salts. Alkanediazonium salts being very reactive cannot be isolated.
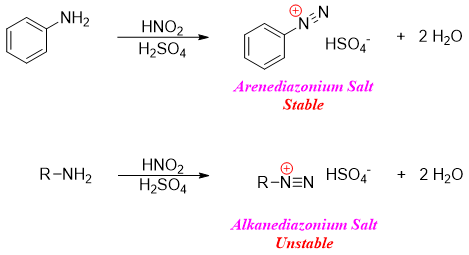
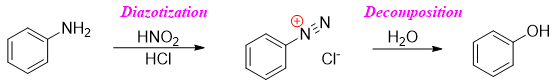
The process of diazotization readily takes place at room temperature. Also, the decomposition of diazonium salt to phenol takes place at room temperature. To overcome the problem of decomposition, the diazotization reaction is carried out at low temperatures. At low temperatures the formation of phenol is suppressed.
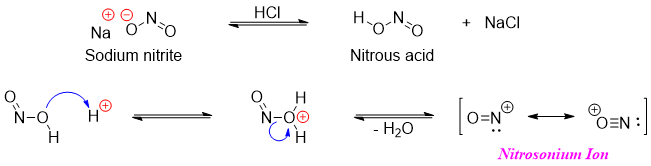
The conversion of amine group (-NH2) to diazonium group (-N2+) requires nitrosonium ion. Nitrosonium ion is generated from nitrous acid (HNO2). Nitrous acid being unstable is commonly generated in situ by treating sodium nitrite (NaNO2) with dilute acid.
Nitrosonium ion being reactive in nature reacts with primary amines to produce diazonium salt.
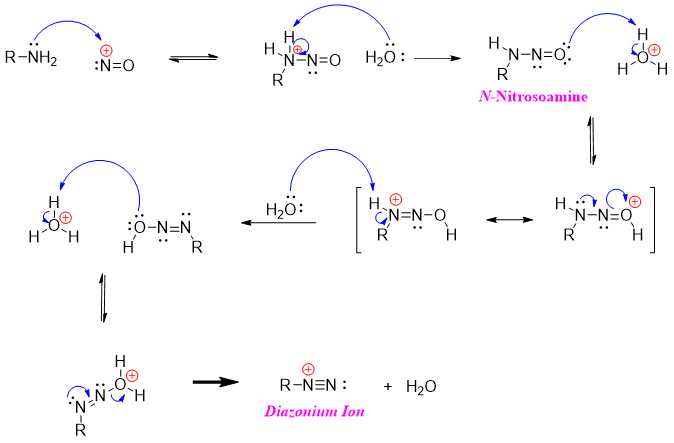
When secondary amines are reacted with nitrosonium ion they produce N-nitrosoamines. Nitrosoamines are stable in nature as they lack N-H proton which tautomerized in mechanism of primary amines shown above.
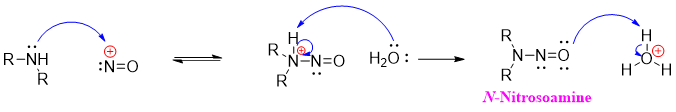
The nitrosoamines formed in the reaction mixture separates as an oily liquid. Preclinical studies have shown that nitrosoamines causes cancer in animals. These findings have caused fear about the ordinary technique of using sodium nitrite as a meat preservative. Sodium nitrite if eaten can react with stomach acid and can lead to the formation of nitrous acid. The nitrous acid can react with amines present in food and convert them into nitrosoamines hence increasing the chances of causing cancer.
