Diazonium Reactions
Diazonium Reactions
There is list of substituents which can be placed on benzene ring using diazonium salts. The reaction is feasible as the leaving group is nitrogen gas (N2) which is very stable.

Following are some known reactions involving diazonium salt conversion into different functional groups.
The Sandmeyer Reactions
The use of Copper (I) salts to replace the diazonium group on benzene ring is called as Sandmeyer reaction. The Cuprous salts can be CuCl, CuBr, and CuCN.
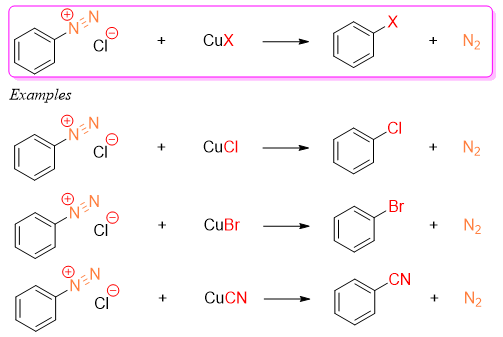
The Sandmeyer rection only takes place by using Cuprous salts. The use of KCl or KBr fails to produce final products. This shows that copper has some special role in the reaction. The exact mechanism is not known for this reaction, but it is believed that the copper ion gives an electron to the diazonium salt making an aryl radical and nitrogen gas.
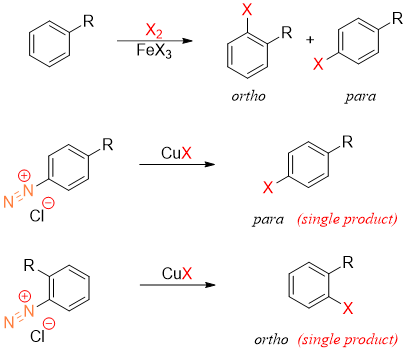
Although, the chlorination or bromination of alkylbenzene ring can be done directly but the diazonium path only gives one product.
Synthesis of Fluorobenzene and Iodobenzene from Diazonium salt
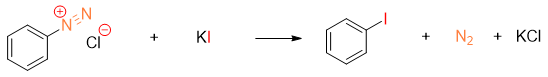
One of the best methods utilized for the synthesis of Iodobenzene derivatives is treating diazonium salt with potassium iodide (KI).
The synthesis of fluorobenzene derivatives from diazonium salt (also called Schiemann reaction) is not an easy reaction thus, this reaction requires some special arrangements. Diazonium fluoroborate precipitates out when diazonium salt is treated with fluoroboric acid (HBF4).
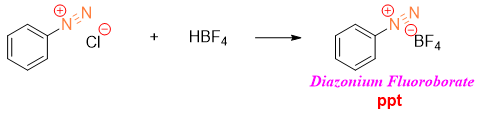
The diazonium fluoroborate precipitate is decomposed to fluorobenzene by heating it carefully.
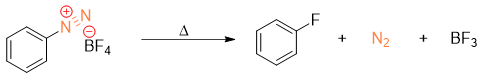
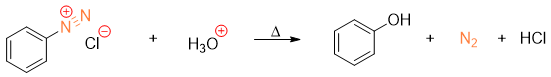
Conversion of Diazonium salt to Phenol derivatives
Diazonium salts when heated with water in the presence of acid (acidic aqueous solution) results in the formation of phenols.
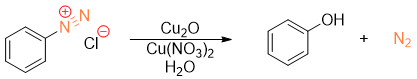
To synthesize phenol from diazonium salt in high yields then diazonium salt is reacted with aqueous Cu2O and Cu(NO3)2 at lower temperatures.
Replacing Diazonium group with Hydrogen atom
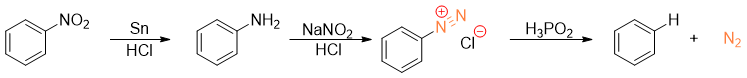
When arenediazonium salt is treated with hypophosphorous acid (H3PO2), the diazonium group will be replaced by hydrogen atom.
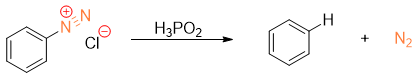
This is very important reaction for the removal of amino group or nitro group from benzene ring. In synthesis, amino and nitro groups are often placed to direct the incoming substituents on benzene ring. Once their role is completed, they can be removed by converting them into diazonium salt and then treating them with H3PO2 to substitute it with hydrogen atom.