Conjugate Addition of Dienes
Conjugate Addition of Dienes:
Conjugated dienes undergoes reactions similar to that of alkenes.
They give two types of addition reactions:
1, 4-Addition Reactions
1, 2-Addition Reactions
Conjugate Addition:
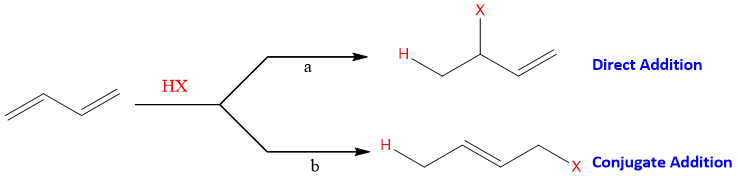
The addition reaction of diene in which electrophilic part and other part of reagent add to 1 and 4 position of the diene is called as conjugate addition. This addition reaction is also called as 1, 4-addition.
Direct Addition:
The addition reaction of diene in which the reagent add on the adjacent carbons of a double bond i.e. at 1, 2 positions is called as direct addition. This addition reaction is also called as 1, 2-addition.
Explanation: Consider the addition of HX (hydrogen Halide) to 1, 3-butadiene. The addition can be carried out in two ways given as;
Mechanism:
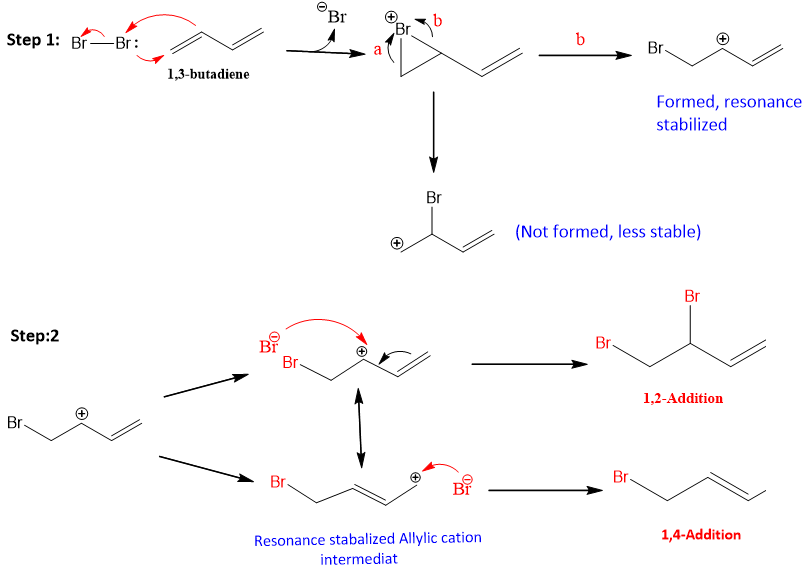
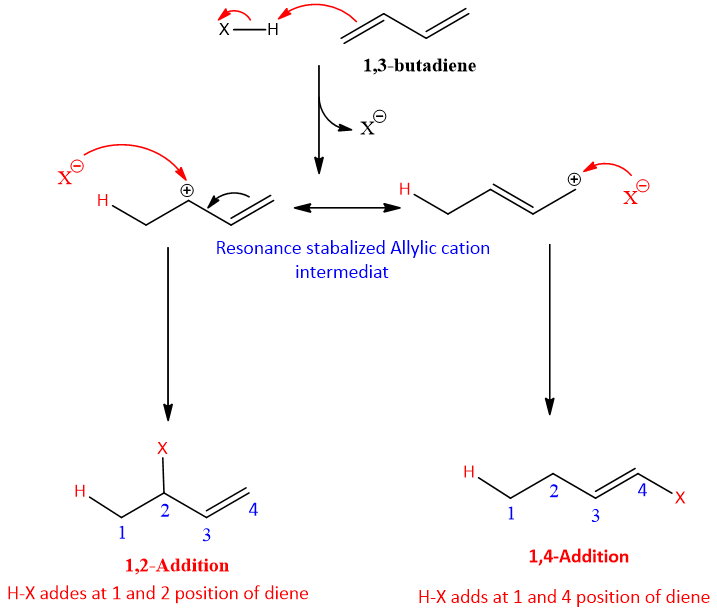
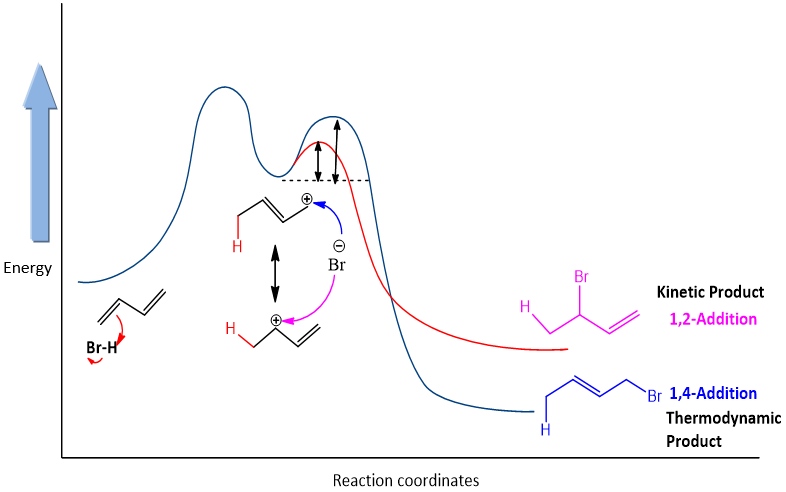
The mechanism for the addition reaction of dienes is different from simple dienes. The first step is similar in 1, 2 and 1, 4-additions; it involves the formation of an allylic cation as an intermediate.
Step 1: It is similar to the first step of addition reaction of alkenes. Electron density of pi electron attack on the hydrogen atom and halide ion leaves.
Step 2: This step is different from the addition reaction in alkenes. An allylic cation is formed and this cation is resonance stabilized. One of the resonance forms has positive charge on second carbon and one has on fourth carbon. Therefore, halide ion can attack on both these positions and two different products are formed.
Conditions for conjugate and direct addition:
The product formed by the conjugate addition is formed due to the stabilization of allylic cation. So, this product is more stabilized and formed at higher temperature. Thus, the product is called as thermodynamic product and conditions are called as thermodynamic conditions.
The product formed by direct addition is called as kinetic product because it forms faster than other product. It occurs at lower temperature.
Consider the energy profile and reaction of HBr with butadiene. Under different temperatures it gives different product. At lower temperature, the relative amount of products formed by two reactions is determined by rate at which these two additions take place. At a lower temperature of about -80 , kinetic product is formed i.e., product by 1.2-addition. It is due the reason that the energy barrier to form the product is very less for 1,2 addition as compared to conjugate addition.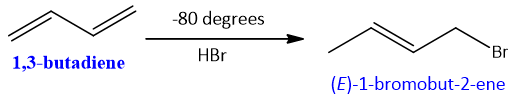
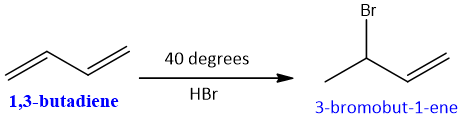
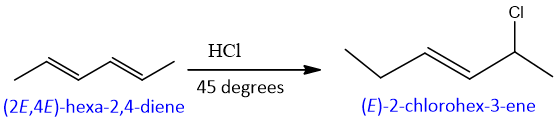
Some Examples of conjugate and direct Additions:
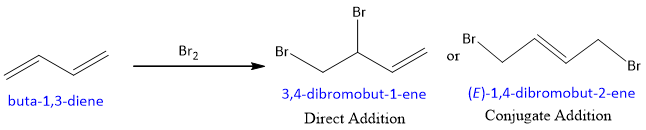
Halogen adds to conjugated dienes by the same two modes as hydrogen halides add.
Mechanism: