Nomenclature of Dienes
Nomenclature of Dienes:
In IUPAC Nomenclature dienes are named as Alkadienes.
Rules for Nomenclature of dienes:
- 1. Select the longest chain that contains the double bonds.
- 2. The longest chain is numbered such that double bond gets the smaller number.
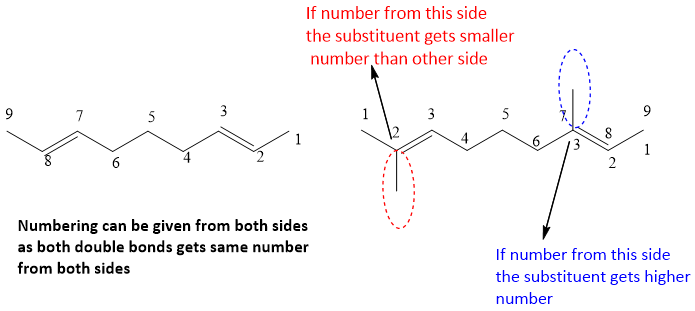
- 3. In case of isolated dienes, if two double bonds get same number from both sides of chain then numbering is given according to other substituents if attached. But if no substituents are present then numbering can be given from any side.
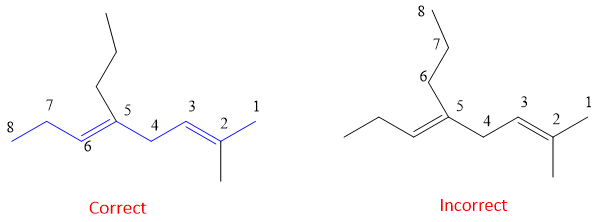
- 4. The parent name is written as ‘Alkadiene’ and written last. Substituents are written in start in alphabetical order e.g. if both methyl and ethyl are present in a compound then in the name ethyl is written first then methyl because e comes first alphabetically then m of methyl group.
- 5. In the parent name positions of the double bonds are mentioned by the number of first carbon of the double bond i.e., that carbon of double bond having lower number. The positions of the substituents are mentioned by the carbon numbers in parent chain to which they are attached.
- 6. The name of substituents and their positions are separated by putting hyphen (-) between the name of substituent and position number.
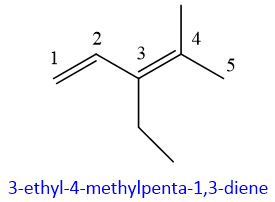
- 7. If same substituent is present more than one time than the number is added by prefixes like di if present two times, tri if present three times, tetra, penta and so on. Positions are also mentioned if same substituent is present more than once. Between the positions of the same substituents comma is added.
- 8. The prefixes are not considered while naming substituents alphabetically.
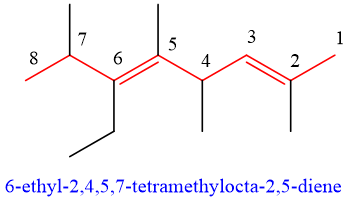
- 9. Geometry of double bonds can also be mentioned if necessary. Geometry as cis/trans or E/Z can be mentioned in the start of name.
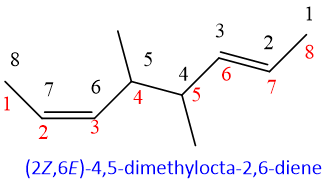
In the above structure, numbering can be possible from both sides, because both double bonds and substituents are same, get same number from either side.
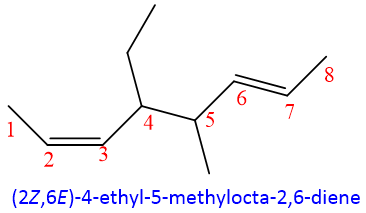
If one of the methyl substituent in above example is replaced by ethyl, then numbering will be given from the side of ethyl as it is preferred alphabetically instead of methyl group.
- 10. In case of ring diene system, the protocol for naming is same as for aliphatic dienes.
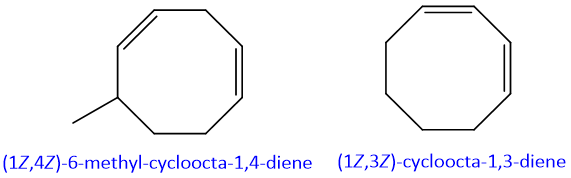
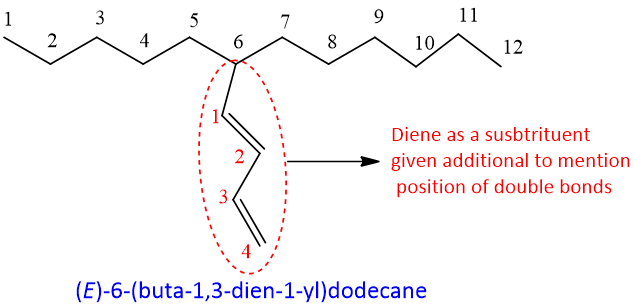
- 11. If the both double bonds of diene system are not part of longest chain than they are named as substituent. They are named as “alkadienyl”.
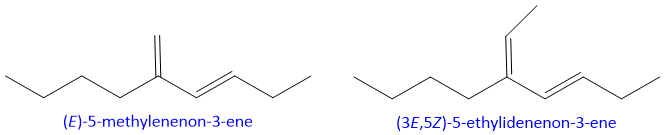
- 12. If one of the double bond of diene system is part of longest chain and other double bond is attach to carbon of longest chain but not the part of longest chain than it is named as Alkylidene ( from alkylene e.g. ethylene, butylene etc.)
- 13. If both double bonds of diene are not part of longest chain and one double bond is attach to carbon of longest chain than they become substituent and are named as ‘allylidene’.
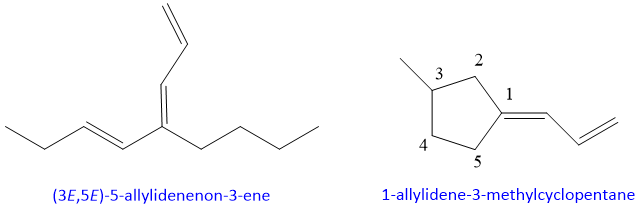
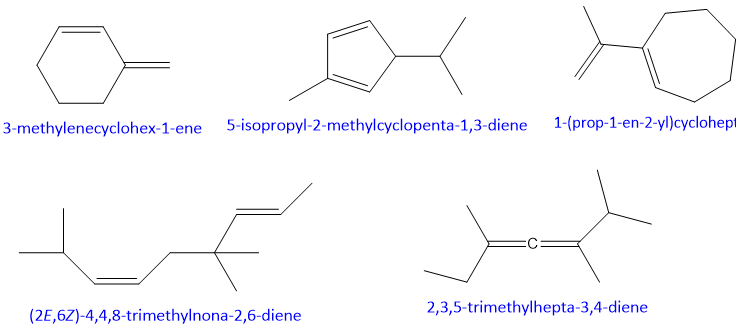
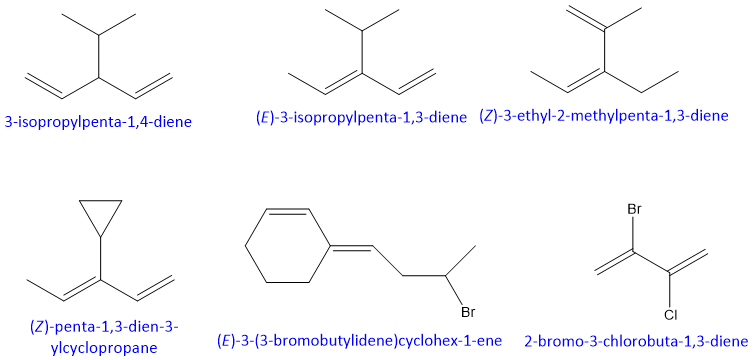
Examples: