Conjugate Substitution
Conjugate substitution:
The substitution that takes place through conjugation or resonating a pi-bond is called as conjugate substitution.
Explanation:
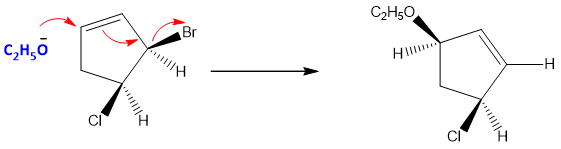
If the substrate is an allylic halide, then there is a tendency of Nucleophile to attack on the carbon of allylic double bond rather than to attack directly the allylic carbon to remove halide ion (leaving group).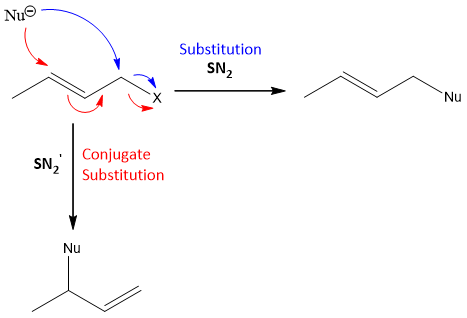
So, if an allylic halide is present, the product can formed by the rearrangement of double bond.
As the reaction is single step, so the reaction is called as SN2’ (‘means conjugation). For this reaction to occur the carbon attach to halide must be bulkier. So, that the Nucleophile finds it difficult to attack on this carbon and it attacks on the carbon of double bond.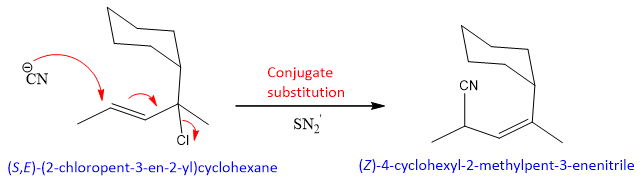
The factor that allows this reaction to occur is the formation of more substituted and stable double bond in the product. However this reaction can compete with simple SN1 reaction because chlorine is attach to a tertiary carbon, which can results in the formation of tertiary cation. On the cation then Nucleophile attack.
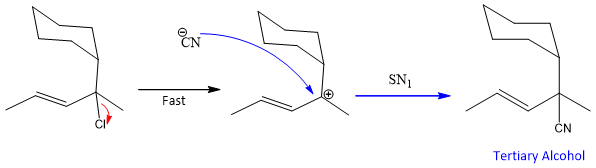
But the major product formed by conjugate addition. It is because of another reason that the rearrangement can also takes place on the tertiary cation. Because the cation formed is resonance stabilized and has two resonance forms.
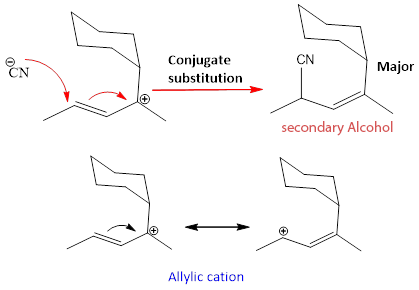
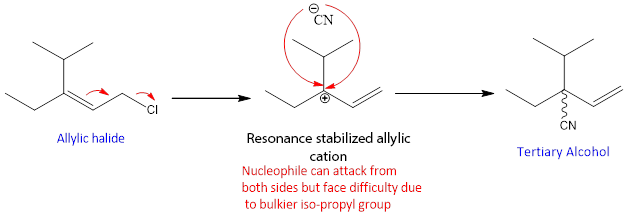
In this case, allylic cation can form but Nucleophile finds it difficult to attack on the tertiary cation position due to the presence of bulkier groups that create steric hindrance. As this conjugate substitution involves SN1 mechanism but with rearrangement so it called as SN1’. But the product of this conjugate substitution is not dominating due the steric factor explained above another product is formed which is the major product.
The formation of major product with bulkier groups on allylic double bond is given as;
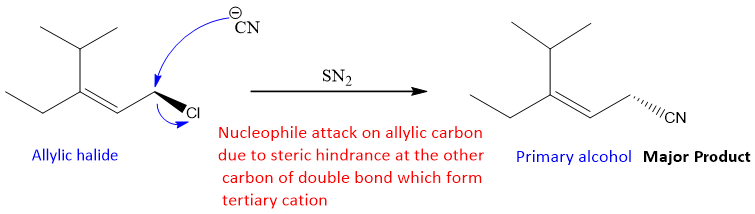
So, from the above examples and explanation it is concluded that to do successful conjugate substitution;
- Allylic carbon of allylic halide must have some bulkier alkyl groups attach to it instead of hydrogen i.e., secondary or tertiary halide.
- The double bond of allylic halide should not have any sterically hindered groups attached to it.
- A good leaving group like iodide, tosyl group can also increase the rate of conjugate substitution because they can leave and assist in the formation of cation.
But when the Nucleophile is carbon these conditions may change. For example consider the conjugate substitution in the presence of organo copper compounds.

Copper metal form complex with the double bond and SN2’ substitution takes place. The major product formed is through conjugate substitution because copper compounds favor conjugate substitution. The most probable mechanism is shown below;

Similarly, when the halide is secondary the major product is still formed by rearrangement, no matter if the product has orientation of stable double bond or not. For example,
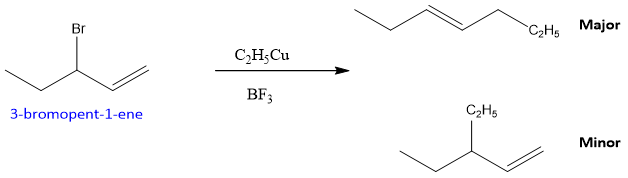
The mechanism followed by this reaction is given below;
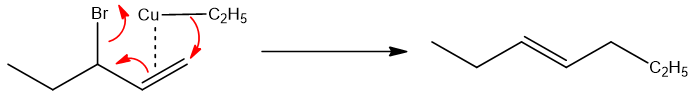
Hence, it is concluded that in case of Organo-Cu compounds as a catalyst like Gillman reagent, the dominating product is formed by conjugate substitution, no matter the halide is primary or the product will have less substituted product.
Stereochemistry:
The product formed by SN2’ reaction, have retained configuration. This is due to the equivalent resonance forms of allylic cation which creates same faces on both ends of allylic system, therefore when the Nucleophile attacked a face it will be the same as to which the halide is attach.