Diels Alder Reaction
Diels Alder reaction:
A cyclo-addition reaction, takes place between a conjugated diene and an alkene or 2π electron system and resulted in the formation of a six-membered ring system, is called as Diels Alder reaction. Otto Diels with his research student Kurt Alder discovered this special pericyclic cyclo-addition reaction in 1928 during their working at University of kiel. They were also awarded with a Nobel Prize for this reaction in 1950.
Characteristics:
- It is a special class of cyclo-addition reaction also called as 2π + 4π cyclo-addition reaction
- The reaction is concerted i.e. it is a one-step reaction.
- It does not require any catalyst.
- It is thermally favorable i.e. it takes place on heating.
- The reaction is stereospecific.
- The product of this reaction is always a ring system and as it is the result of an addition, therefore called as cyclo-adduct (ad-from addition and duct-from product).
- The simplest example of diels alder reaction is between 1,3-butadiene and ethane that results in the formation of cyclohexene.
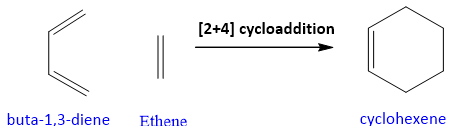
Mechanism of Diels Alder Reaction:
The mechanism is concerted and takes place in the presence of heat. Conjugated Diene is the electron rich system and it attacks on one of the carbon of alkene which is the electron deficient system also called as dienophile.
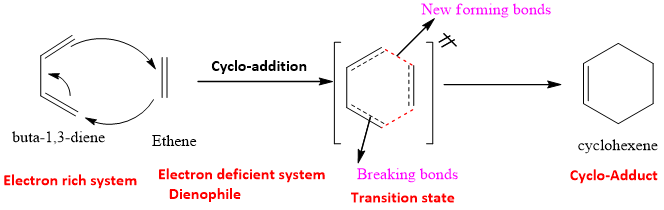
The transition state of this reaction is a six-membered ring and as there are 6 pi-electrons in transition state; it acquires some sort of stability of benzene as it becomes aromatic in nature. This is also a reason for Diels Alder reaction to happen successfully.
Factors Affecting Diels Alder Reaction:
Nature and reactivity of Diene:
Diene in Diels-Alder reaction should be electron rich. For substituted diene any substituents attach to it should increase the electron density on it.
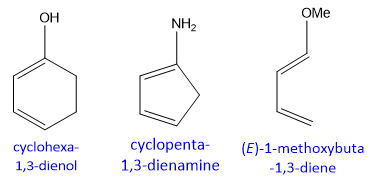
For example, if electron donating groups (EDG) are attached to diene they would increase the electron density on it, therefore, its reactivity increases as compare to un-substituted diene.
Similarly, if substituents attached are electron withdrawing (EWG) in nature they would decrease the electron density on it and it becomes less reactive as compare to simple diene.
Substituent effect on the reactivity of diene towards Diels Alder reaction is shown below;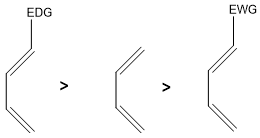
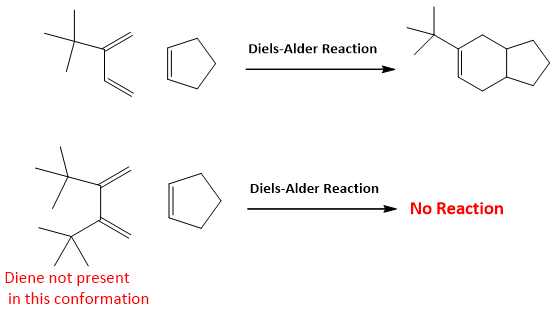
Geometry of Diene:
- Geometry of diene is very important in Diels-Alder reaction. As the diene can exist in two forms i.e. Cis or Trans. For Diels Alder reaction to occur diene should be present in s-cis (cisoid) conformation not in s-trans. Although s-trans conformation is more stable as the two groups are opposite to each other as compared to the s-cis conformation in which two groups are on same side and cause instability due to steric hindrance. But the conversion of s-cis conformation to s-trans conformation requires rotation about the single bond and can takes place at room temperature (about 30KJ/mole energy is required, available at room temperature). So, diene like butadiene can rotate fastly to s-cis conformation which is more reactive and less stable and this is also a reason for successful completion of Diels Alder reaction.
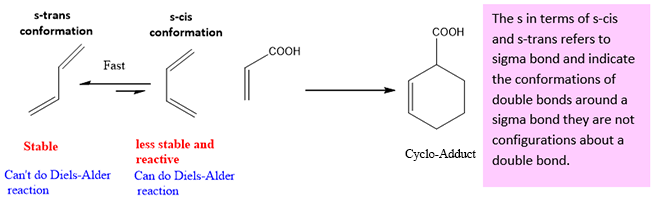
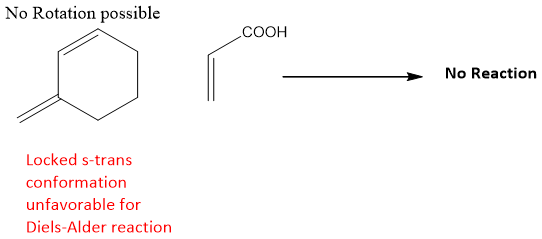
But if the diene has locked trans conformation, it does not undergoes Diels-Alder reaction because it is unable to rotate due this locked conformation.
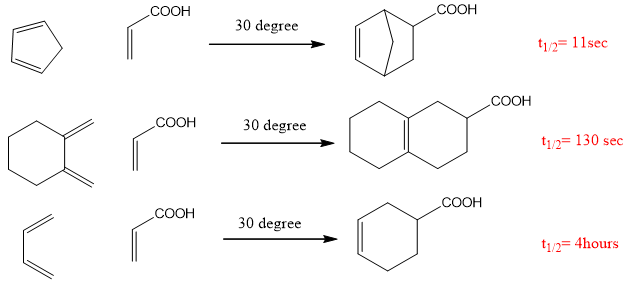
- Cyclic dienes are best for Diels Alder reactions as they have locked s-cis configuration e.g. cyclo-pentadiene is a cisoid diene. The reactions which involve cyclic dienes are faster than others.
Nature and reactivity of Dienophile:
The Dienophile is the electron deficient system and the substituent attached to it must be electron withdrawing in nature. The electron withdrawing groups decrease the electron density on dienophile and make it more electron deficient, therefore more reactive. More electron withdrawing groups attach to the dienophile more reactive it is.
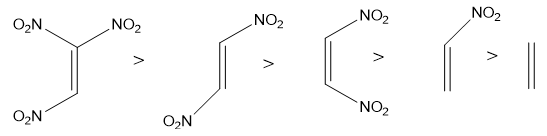
The effective dienophiles for Diels-Alder reaction are shown below
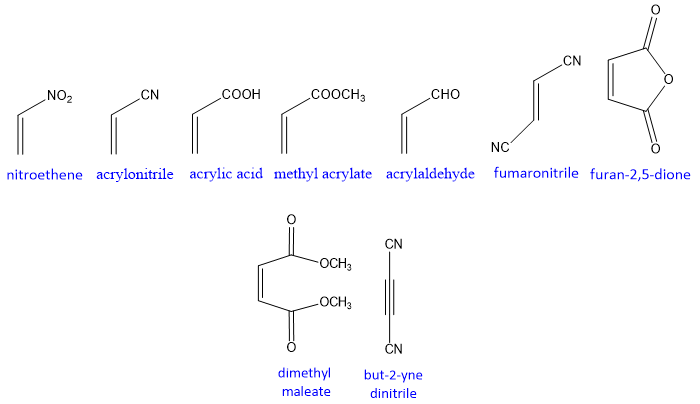
An alkyne can also be act as a dienophile.

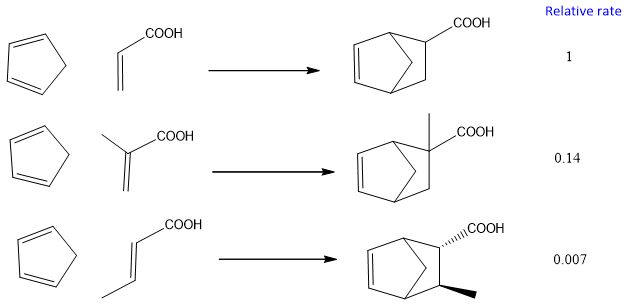
Steric Effect:
Steric hindrance of groups attach to dienophile also effect the rate of reaction. If bulkier groups attach to the dienophile, they becomes less reactive towards the Diels-Alder reaction. For example, cis dienophile has more steric hindrance because the alkyl groups are on similar sides. It decrease the rate of reaction. On the other hand, Trans dienophile reacts fastly. The effect of steric hindrance on the rate of reaction is shown below;
Effect of electron donating and withdrawing groups on product formation:
EDG and EWG not only affect the reactivity of diene and dienophile but they also effect position of substituents on the product. Consider the following reaction;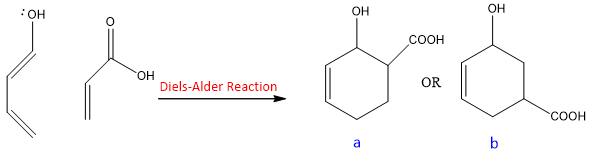
In this reaction, product a and b can be formed. But only one product is formed due to the effect of EWG and EDG on their respective reactants.
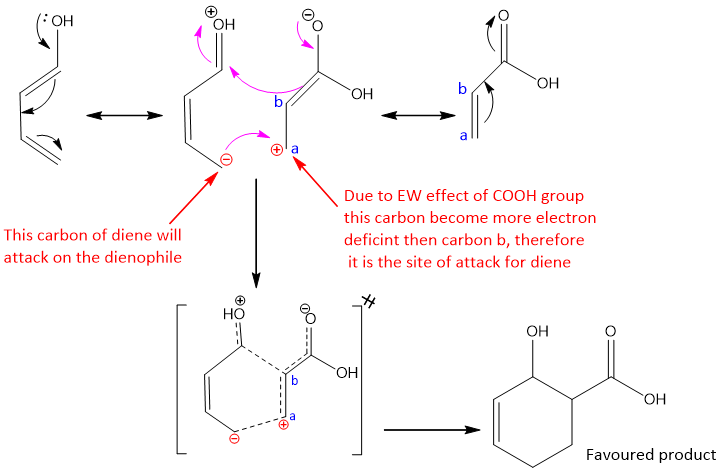
Stereochemistry of product:
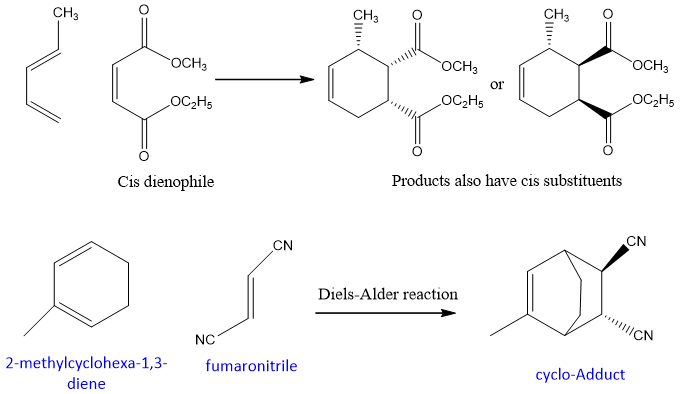
The Diels Alder reaction is a stereospecific reaction which takes place through syn addition. The stereochemistry of dienophile is retained in the product. If the dienophile is cis, in the product substituent attached to it will be cis and vice versa.
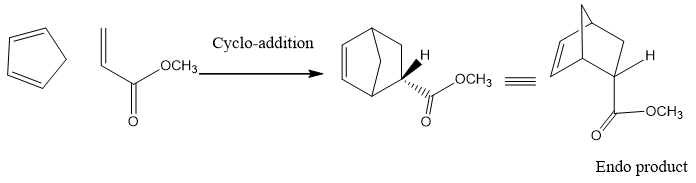
- Now from both these two cis products one has substituents above the plane (above the newly formed ring), this product is called as exo product.
- The other has substituents below the plane (below the newly formed ring) , this product is called as endo product.
- Two factors determine which product is favored;Steric factor and electronic factor.
- According to steric factor, exo product is favored because in this product substituent of both reactants are away from each other. Therefore, they offer less steric hinderance and it becomes stable.
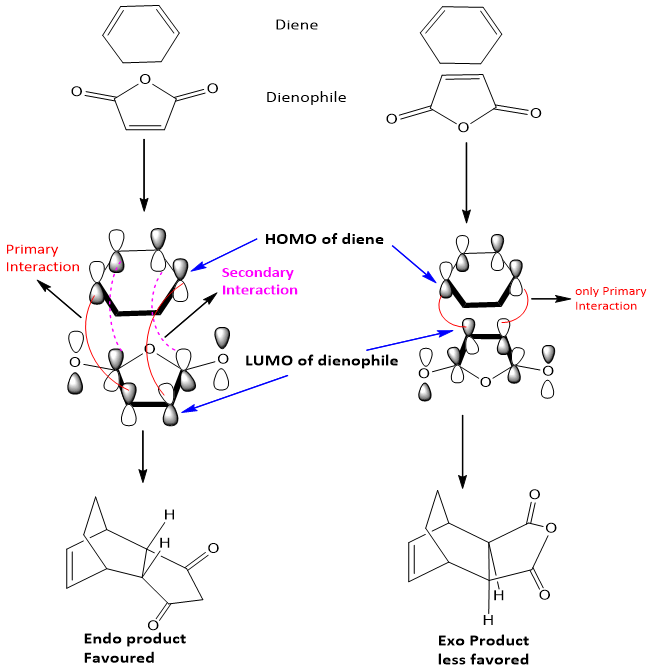
- Electronic factor favors the formation of Endo product. As the EWG on dienophile has also affinity for electron density so it tends to interact with the diene and the electron withdrawing substituents fastly moves to the down the ring position.
- Endo is kinetic product and exo is thermodynamic product.
- Both factors propose different results, but electronic factors dominate over steric factors and thus endo product is favored.
The molecular orbital treatment of transition states formed in both products shows that HOMO of diene and LUMO of dienophile interact with each other. This interaction is called as primary interaction. But in endo product formation another interaction called secondary interaction also develop between the EWG of dienophile and diene. Due to this secondary orbital interaction endo products formation is favored. The primary and secondary interactions can be shown as follows;
Another example of Diels-Alder reaction;