Properties of Dienes
Properties of Dienes:
Physical Properties of dienes:
- The simplest diene is butadiene, it is colorless gas.
- All dienes have characteristic odor like hexadiene has petroleum like odor and butadiene has aromatic odor.
- Larger dienes are insoluble in water except butadiene which can slightly solublize in water. As the dienes are non-polar in nature, they are soluble in non-polar solvents mostly like diethyl ether, chloroform, hexane etc.
- The physical state of dienes changes as the size increases. For example butadiene is gas while hexadiene is colorless liquid and dienes with larger size will be solid.
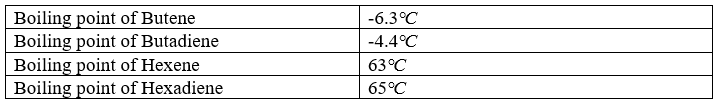
- Dienes have London dispersion forces (instantaneous dipole induced dipole forces) between the molecules. These forces become stronger in larger molecules and affect the boiling point, melting point, and density of dienes.
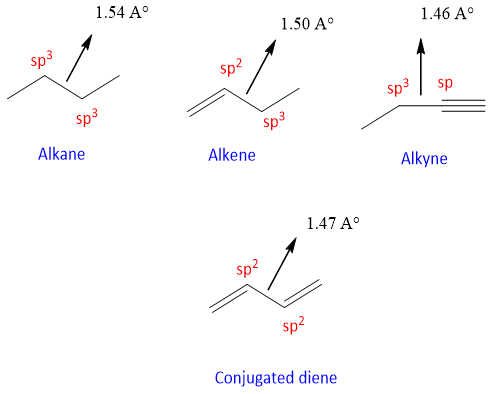
- As a single bond is present between two double bonds, this single bond has a bond of 1.47which is present between the single bond having sp2 and sp3 carbon, and single bond between sp and sp3 carbons as shown below;
- They usually have less density then water. The density of dienes increases as the size increases.
Chemical Properties of dienes:
- Dienes have non-polar nature.
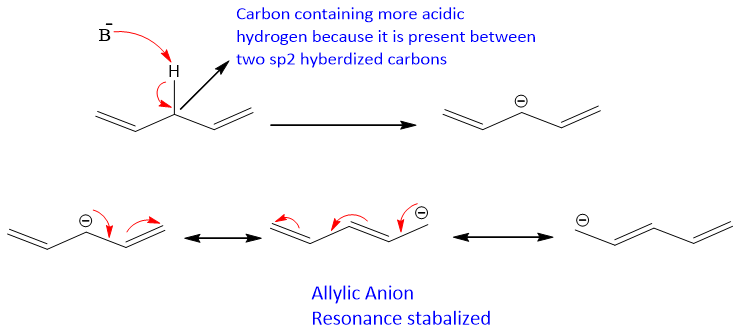
- Among all dienes conjugated dienes shows some aromatic properties due to conjugation.
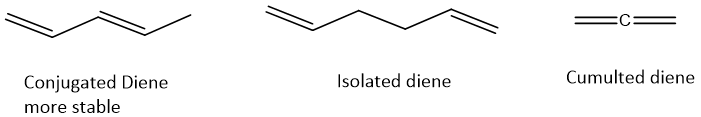
- All dienes have sigma and pi-bonds. Due to presence of pi-bonds dienes can show a number of reactions. They can undergo addition reactions, both nucleophilic and electrophilic. They can undergo oxidation reactions as well.
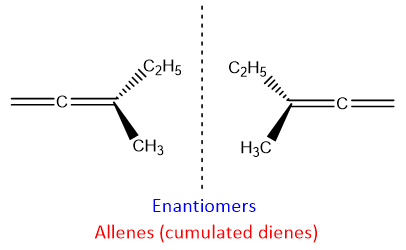
Cumulated dienes are chiral in nature. Although they don’t have chiral carbon. But asymmetry is developed in the molecule if two different substituents are attached on the carbon of cumulated diene. Moreover, planarity is also lost in the molecule; both double bonds are present perpendicular to each other due to which one double bond and substituent attached to this carbon are planer. On the other hand, the other double bond and substituents attached to it are not planer, they are arranged in space. This arrangement creates chirality in the molecule. Therefore, cumulated dienes exist as enantiomers.