Stork Enamine Reaction
STORK-ENAMINE REACTION
You may have encountered the formation of enamines in a previous article. To help you recall about what enamines are, they are produced from the reaction of an aldehyde or ketone and a secondary amine.
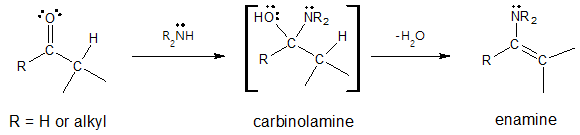
So given that we already how to produce enamines, what’s next for the said compound? Or we can simply ask ourselves, “what’s the importance of enamines in organic chemistry or synthesis?”
We can appreciate the importance of enamines when considering the alkylation of carbonyl compounds. The dilemma is that when one wants to introduce alkyl groups to a carbonyl compound such as ketone, the following problems could be encountered:
- i. if the product formed still has H atoms in the alpha carbon (carbon next to the C=O group), it can keep on forming enolates (recall keto-enol tautomerism) to get more alkyl groups added:

- ii. if one starts with asymmetric ketone, then two possible enolates can form, which also lead to mixture of alkylated products
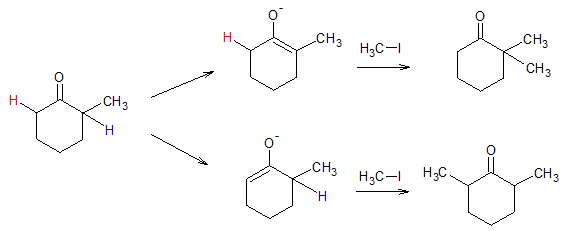
two possible enolates can be formed from asymmetric carbonyl compound
So as a remedy to the problems encountered with reaction involving enolates, Gilbert Stork developed the process of enamine alkylation. The method simply suggests that instead of starting directly with a ketone, why not convert it first to an enamine which you have gained a background already from previous articles. With enamine, it reacts selectively to give mono-alkylated product and the resulting compound would also be regioselective. When alkylation is done, the amine group can be hydrolysed off to regenerate the starting ketone. The overall reaction is shown below.
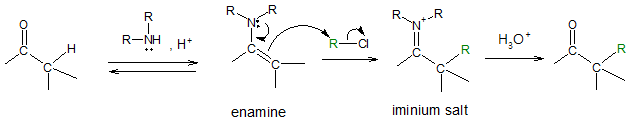
The commonly used secondary amines to form the enamine are cyclic ones like pyrrolidine.

Just a summary of the steps involve in Stork enamine reaction:
- Form the enamine;
- Allow the enamine to react with electrophiles;
- Get rid of the amine via hydrolysis.
The mechanism of the transformation show above via Stork-Enamine reaction is illustrated here:
Mechanism:
- 1. Enamine formation:

- 2. Enamine reaction:

- 3. Removal of amine group via hydrolysis:

Despite the advantage of the above process, enamines have intermediate reactivity with respect to enol and enolates. That is, enamines are more reactive than enol, but less reactive than an enolate ion. The advantage of reactions via enamines over through enolates is that the former occur under milder conditions than the latter, suggesting that there is a lower chance for side reactions to occur.
And that’s it for Stork-Enamine reaction.
