Cyanohydrin Formation
Cyanohydrin Formation
Cyanohydrin formation, also known as nucleophilic addition of HCN, is a perfect example of base-catalyzed addition of HCN to a carbonyl group. Hydrocyanic acid, HCN, is water soluble and mildly acidic whose conjugate base is a strong base and a strong nucleophile. It attacks aldehydes and unhindered ketones to give addition products called cyanohydrins.
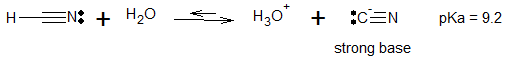
The mechanism of the base-catalyzed nucleophilic addition, is shown below
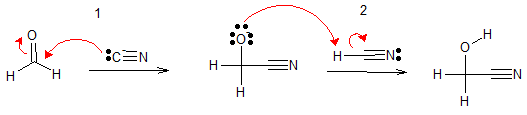
(1) The nucleophilic cyanide ion attacks the carbonyl group and yields an alkoxide ion. (2) The alkoxide ion is then protonated to give the cyanohydrin. Formaldehyde reacts quickly and quantitatively with HCN. Most other aldehydes have equilibrium constants that favor cyanohydrin formation.
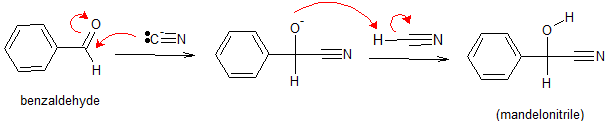
Let’s take a look at another example. Following the mechanism shown above, addition of nucleophilic cyanide ion to benzaldehyde will give mandelonitrile.
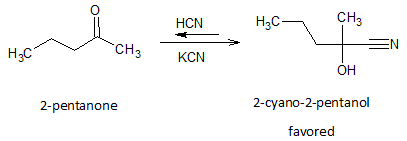
Reactions of HCN with ketones have equilibrium constants that may favor either the ketones or the cyanohydrins, depending on the structure. Ketones that are hindered by large alkyl groups react slowly with HCN and give poor yields of cyanohydrins. Cyanohydrins may also be formed using liquid HCN with a catalytic amount of sodium cyanide or potassium cyanide.
For example, in the nucleophilic addition of cyanide to 2-pentanone (unhindered ketone), the reaction will give high yields of 2-cyano-2-pentanol.
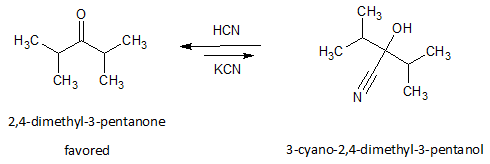
While nucleophilic addition of cyanide to 2, 4-dimethyl-3-pentanone (hindered ketone) will yield only very small amount of cyanohydrin product.
General reactivity trend of ketones and aldehydes are as follows;
Formaldehyde > other aldehydes > unhindered ketones > hindered ketones
