Reductive Amination of Aldehydes and Ketones
REDUCTIVE AMINATION OF ALDEHYDES AND KETONES
There are a number of available methods that one can utilize in order to make amines. Particularly in this article, we will focus on the process called reductive amination. The said method involves treatment of an aldehyde or a ketone with ammonia or an amine in the presence of a reducing agent. Depending on the desired amine to be synthesized, nitrogen compounds ammonia, primary amines and secondary amines can be used producing primary, secondary and tertiary amines, respectively.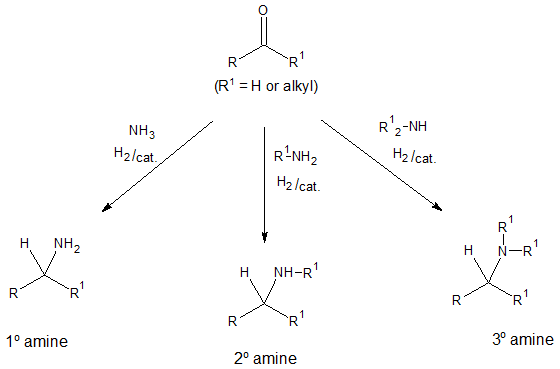
Before going to specific examples, let’s look at first the mechanism involved in reductive amination. Regardless of the type of amine produced, all undergo the same reaction pathway and is shown in the next diagram. As an overview, an imine intermediate is first formed via nucleophile addition reaction and the C═N bond of the imine is then reduced.
Mechanism of Reductive Amination
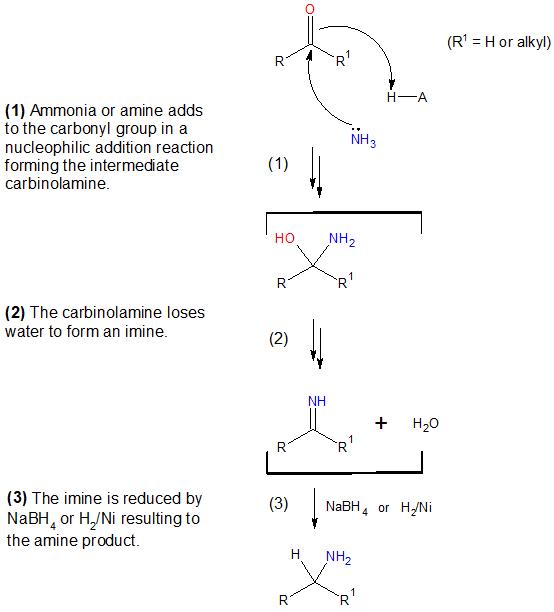
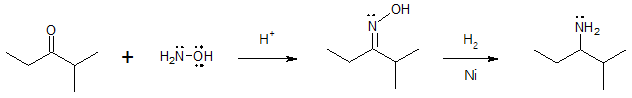
Aside from the reducing agents indicated in the given mechanism, other reducing agents could also be used. Commonly used reductant is sodium cyanoborohydride, NaBH3CN which has a similar reactivity with that of NaBH4 but the former has a greater stability in acidic medium. Specific examples are shown in this article illustrating how NaBH3CN and other reducing agents work in producing amines from aldehydes and/or ketones. In preparing primary amines via reductive amination, ammonia is seldom replaced by hydroxylamine since the oxime intermediate formed is more stable and can be easily isolated prior to reduction step.
Formation of a tertiary (3o) amine:
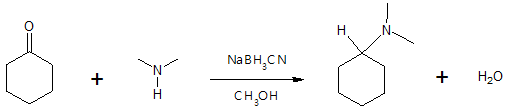
Formation of a secondary (2o) amine:
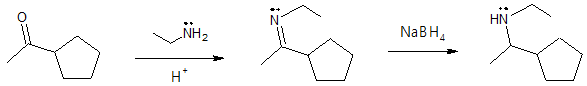
Formation of a primary (1o) amine: