Nomenclature of Aldehydes and Ketones
NOMENCLATURE OF ALDEHYDES AND KETONES
Most of organic chemistry involves the chemistry of carbonyl compounds. Aldehydes and ketones, in particular, are intermediates in the production of pharmaceutical agents, biological pathways and many industrial processes. Thus, understanding its properties and reactions is essential. Before we look into the important reactions of aldehydes and ketones, let’s take a peek first about the nomenclature of the said carbonyl compounds.
Naming Aldehydes
Based on IUPAC rules, aldehydes are systematically named by replacing the final –e of the alkane name with –al. It is important to note that the parent chain (the longest continuous carbon chain) must contain the –CHO group and that the carbon in the –CHO is numbered as carbon 1. Look at the examples below.

Recall that groups not part of the parent chain are considered as substituents, hence, in the second example above, carbon 2 has isopropyl group which is mentioned first in the complete IUPAC name followed by the name of the parent aldehyde.
Now if the –CHO group is attached to a ring, then the suffix carbaldehyde is used instead of changing the –e to –al.
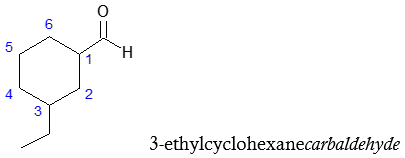
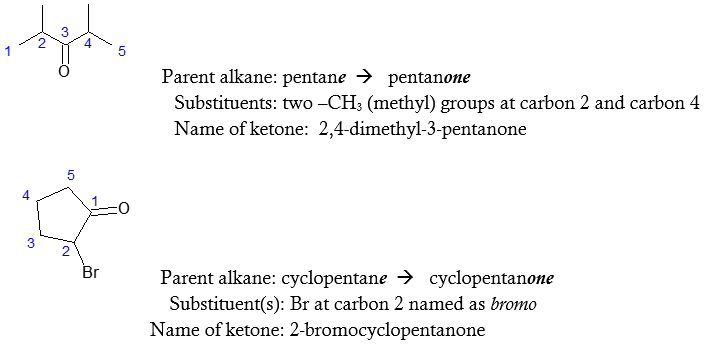
Naming Ketones
Ketones are named by replacing the terminal –e of the corresponding alkane name with –one. Like in the case of aldehydes, the parent chain is the longest one that contains the ketone group (-C=O). In terms of numbering the parent chain, the numbering must start at the end nearer the carbonyl carbon. Since the position of the -C=O may vary in the molecule, the locant of the carbonyl carbon must be indicated in the IUPAC name. Examples are illustrated below.

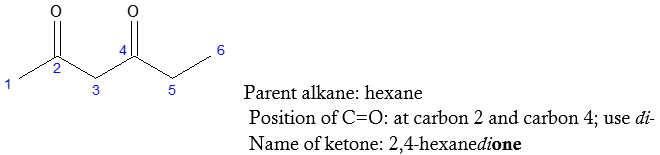
How about if there are more than one –C=O present in the compound? The adjustment is to use the appropriate prefix paired with the ending -one indicating the number of carbonyl groups in the molecule such as di-, tri-, tetra-, etc. In addition, the name of the corresponding parent alkane is retained without changing the ending –e anymore.
Aldehyde and Ketone groups as Substituents
When a molecule contains a functional group with higher priority than aldehyde or ketone group, the –CHO and –C=O groups will be considered as substituents. To help you recall the order of priority of most of the functional groups, refer to the table below listing the different functional groups in decreasing order of priority starting with carboxylic acids.
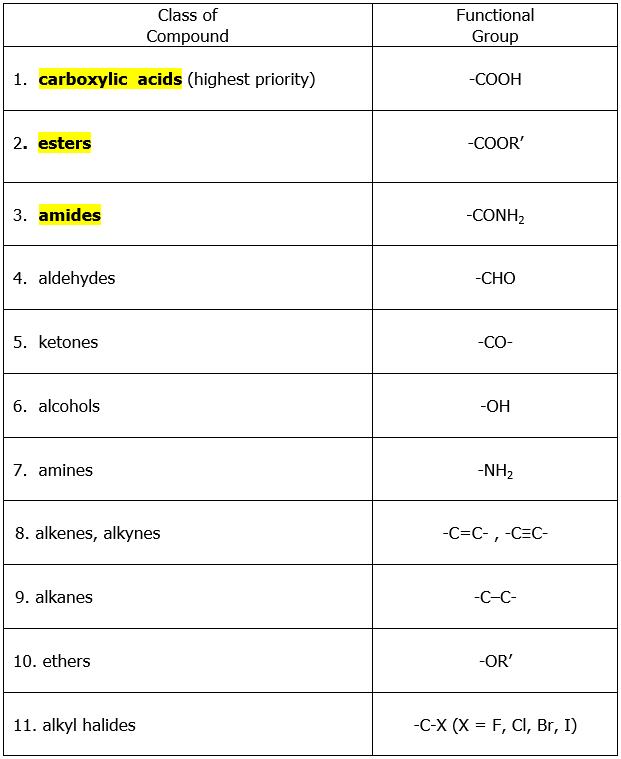
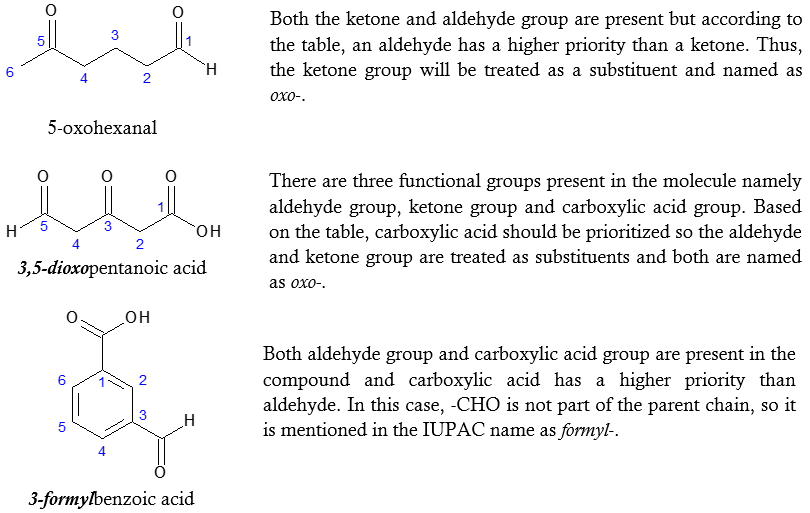
The functional groups highlighted in the above table, when present in the molecule together with either aldehyde or ketone group will make the latter as substituents. The carbonyl group (-C=O) of the aldehyde or ketone group is named by the prefix oxo- if it is included as part of the longest carbon chain (parent chain). When –CHO group is not part of the longest chain and is a substituent, it is named as formyl-. For a clearer picture of the said rules, look at the examples below.
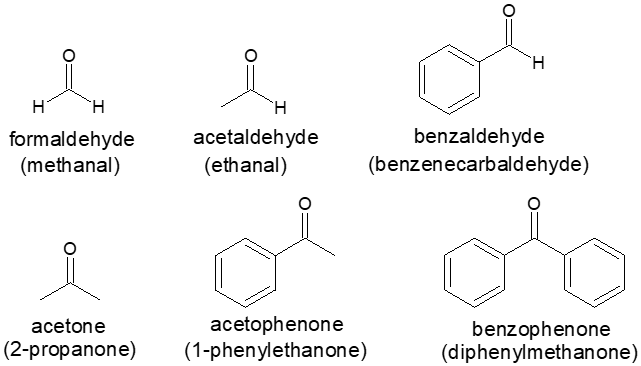
Aldehydes and Ketones with Common Names
A few simple and well-known aldehydes and ketones have common names that are recognized by IUPAC. In this case, it would be an edge in taking organic chemistry to be familiar with them. Some of these carbonyl compounds are presented below with their IUPAC name enclosed in parenthesis.