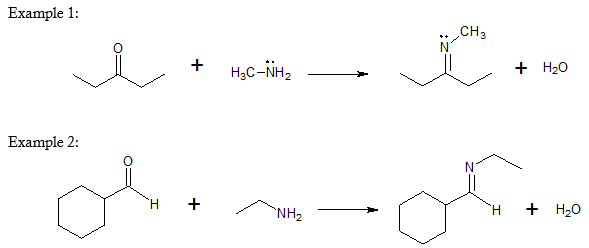
Formation of Imine
FORMATION OF IMINE
Another characteristic reaction of aldehydes and ketones is the nucleophilic addition of organic nitrogen compound such as ammonia and amines. Each type of amine reacts differently with either aldehyde or ketone. Before we go into the reaction proper, let’s review for about the classification of amines.
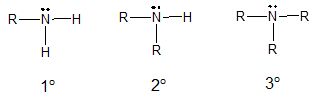
Illustrated above are the three major types of amines namely primary (1o), secondary (2o) and tertiary (3o) amines. Each type differ in the number of carbon or alkyl groups bonded to the nitrogen atom. Primary amines only have one carbon group bonded to the nitrogen atom of the amino group. Secondary and tertiary amines have two and three alkyl groups, respectively. In relation to imine formation, the reaction involves the addition of primary amines to aldehydes and ketones.
Nucleophilic Addition of Primary Amines
Primary amines, RNH2, add to aldehydes and ketones to yield imine, R2C=NR.
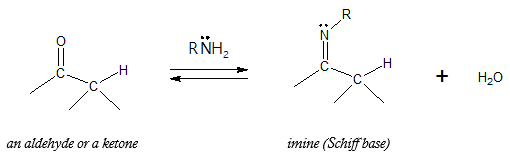
Imines are considered as nitrogen analogues of aldehydes and ketones in the sense that the carbonyl group (C=O) is replaced by a carbon-nitrogen double bond (C=N). Just like amines, imines are basic also referred as Schiff base. Notice from the above reaction that imine formation is another example of condensation reaction, a reaction that joins two or more molecules accompanied by a loss of a small molecule such as water. With the general reaction shown above, it can be seen that an imine is formed by replacing the O of the C=O group with N-R of the RNH2 while the lost O is combined with the H atoms of the primary amine, forming H2O. To have a better look at how the imine is formed from the starting aldehyde or ketone and primary amine, examine the reaction mechanism shown in the next figure.
Mechanism of Imine Formation:
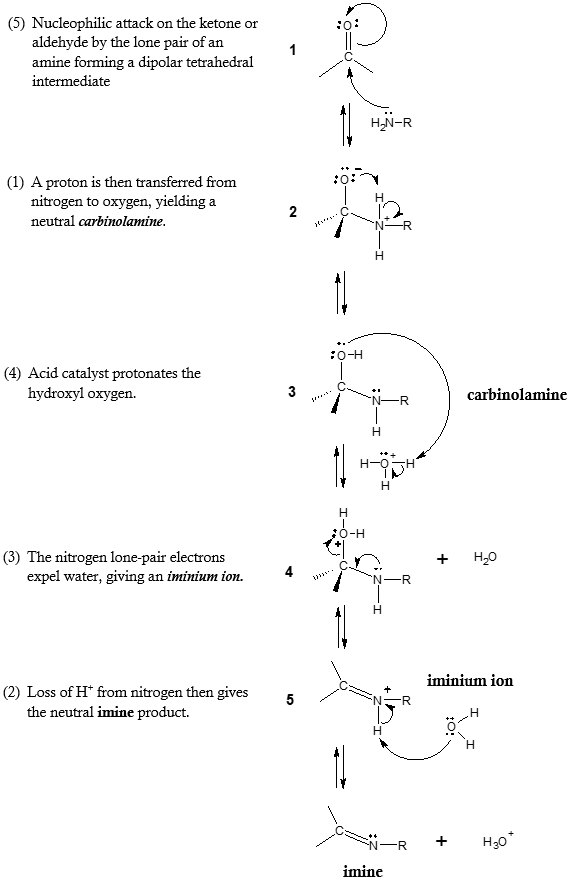
As can be observed from the mechanism, imine formation is a reversible, acid-catalyzed process that begins with the nucleophilic addition of the primary amine to the carbonyl carbon, followed by transfer of a proton from nitrogen to oxygen to yield a neutral amino alcohol or also known as carbinolamine. Protonation of the oxygen atom of the carbinolamine intermediate by an acid catalyst then converts the –OH into a better living group (-OH2+), and an elimination-like loss of water produces an iminium ion. Loss of proton from nitrogen gives the final imine product and regeneration of the acid catalyst.
Another consideration when carrying out imine formation is the pH. The said reaction tends to be slow at both high pH and low pH. It was found out that the reaction has a maximum rate, meaning the reaction is fastest at pH around 4 to 5.
See further examples of imine formation below.