Enolate Ion Formation
ENOLATE ION FORMATION
If you are familiar with keto-enol tauromerism, you may have seen enolate ion already. Enolate ions intermediates of the interconversion between keto form and enol form of carbonyl compounds. The negatively charged enolate ion is formed by abstraction of a hydrogen atom in an α carbon.
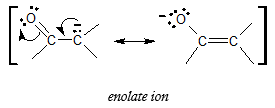
The formation of enolate ion has something to do with the ease of abstraction of the hydrogen atom bonded to the α carbon of the carbonyl compound. The ease of removal of the α proton is related to the acidity and below is the comparison of ease of removal of the α proton of an aldehyde or a ketone with other organic compounds with considerable acidity.
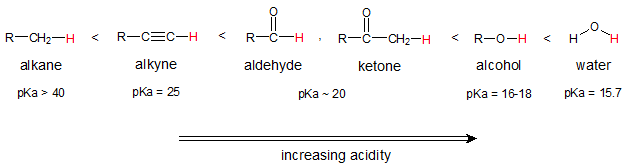
Aldehydes and ketones are much more acidic than alkanes or alkynes but less acidic than alcohols and water. With this degree of acidity (the lower the pKa, the easier to remove the acidic proton), treatment of a simple aldehyde or ketone with hydroxide ion (OH-) or alkoxide ion (OR-) will only produce a small fraction of the deprotonated, enolate ion at equilibrium.
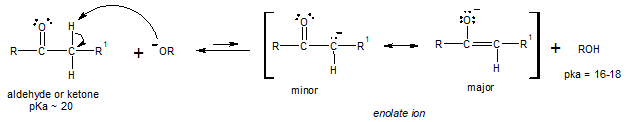
Recall that in an acid-base equilibrium, the reaction favors the direction that yields the weaker acid. This accounts for the left side of the reaction above to be favoured, limiting the enolate ion concentration present at equilibrium.
Nonetheless, the small amounts of enolate ion present at equilibrium can still serve as a reactive nucleophile. We can take advantage of this reactivity of enolate ion by allowing it to react to an electrophile other than a hydrogen ion (H+). When the enolate ion reacts, it is being consumed and thus, decreases in concentration, which shifts the equilibrium position towards the enolate ion formation (recall concentration effects with equilibrium position, governed by Le Chatelier’s principle).
Using Lithium Diisopropylamide (LDA)
When we want to utilize an enolate ion to react with an electrophile in a subsequent step of a synthesis, relying on an equilibrium mixture of the enolate ion and the base would likely be a problem simply because the base (hydroxide or alkoxide) would react faster with the electrophile than the enolate ion. In other words, one would want to produce an enolate ion from an appropriate carbonyl compound (aldehyde or ketone) with approximately 100% conversion. Fortunately, there exist a base that is useful and effective for the said purpose known as lithium diisopropylamide (LDA).
LDA can be produced from the deprotonation of diisoprpopylamine with the help of an alkyllithium reagent.
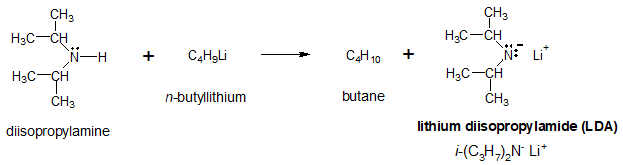
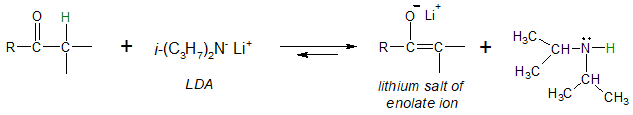
Notice that with the use of LDA, the equilibrium position of the interconversion of the ketone to enolate ion lies far to the right, unlike when hydroxide or alkoxide is used. Lithium salts of enolate ion can be a useful intermediate in the synthesis of a number a organic compounds which will be discussed in separate articles.
Reactivity of Enolate ion
Enolate ions are far more useful intermediates in organic synthesis than enols for two major reasons:
- (1) Stable solutions of enolate ions can be easily prepared from appropriate carbonyl compounds together with strong base such as LDA while enols are short-lived intermediates at low concentrations;
- (2) Enolates are more reactive than enols; enolate ions have negative charge, making them much better nucleophiles than neutral enols
The nice thing about enolate is that we can look at it two ways, and thus, two possible reactive pathway: (1) as a vinylic alkoxide (C═C–O-) or (2) as an α-keto carbanion (-C–C═O). As a result, enolate ions can react with electrophiles either on oxygen or on carbon, depending on where the negative charge resides.
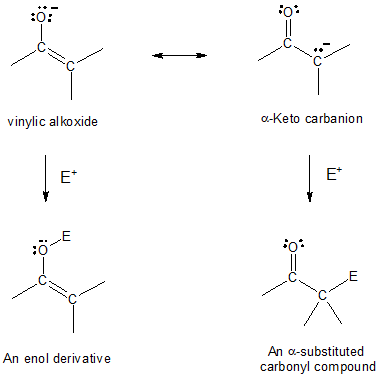
Reactions on oxygen produce enol derivatives while electrophilic addition on carbon yields α-substituted carbonyl compounds. Both reactivities are known but reaction on carbon is more commonly observed.
