Hemiacetals and Acetals
Hemiacetals and Acetals
Formation of Hemiacetals
Aldehydes and ketones can react with an alcohol and form an equilibrium mixture of hemiacetals (acetals of ketones are sometimes referred to as hemiketals). A hemiacetal has a hydroxyl and alkoxyl group on the same carbon. Formation of hemiacetals is an example of a nucleophilic addition of alcohols to aldehydes and ketones. The general reaction is shown below.
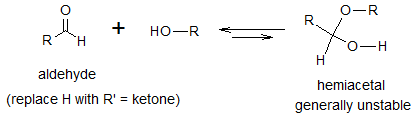
Formation of acyclic hemiacetals are generally unstable. This is because formation of the parent aldehydes (or ketones) and alcohols are greatly favored than the formation of acylic hemiacetals. Difficulty in isolation of hemiacetals are then usually encountered. However the rate of formation of hemiacetals can be greatly increased by the presence of an acid or a base. Below are the mechanism of nucleophilic addition of alcohol to aldehyde or ketone.
Mechanism for Acid-catalyzed hemiacetal formation
Acid catalyst protonates the carbonyl oxygen atom of aldehyde making the carbon in the carbonyl group more electrophilic and much more susceptible to nucleophilic addition of alcohol. Deprotonation with water will then yield hemiacetal.
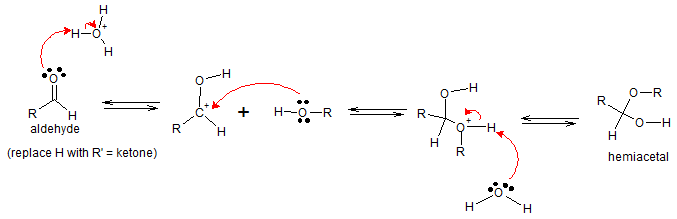
Mechanism for Base-catalyzed hemiacetal formation
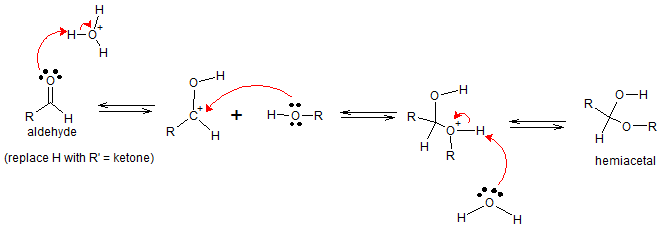
The base catalyst deprotonizes alcohol thereby increasing its nucleophilicity before it attacks the C=O group. Protonation with water will then yield hemiacetals.

There are a few hemiacetals that are stable. They become stable hemiacetals by intramolecular cyclization of aldehydes and ketones to form cyclic five- and six membered ring hemiacetals. Let’s take a look at cyclization of glucose. Cyclization occurs when an alcohol oxygen atom adds to the carbonyl carbon of an aldehyde or a ketone. This happens through the nucleophilic attack of the hydroxyl group at the electrophilic carbonyl group. Since alcohols are weak nucleophiles, the attack on the carbonyl carbon is usually promoted by protonation of the carbonyl oxygen. Stability of cyclic hemiacetals may be associated with stability of the strain free 5-membered ring. Generally, only the most stable (strain free) cyclic structures are produced to an appreciable extent for a given carbohydrate.

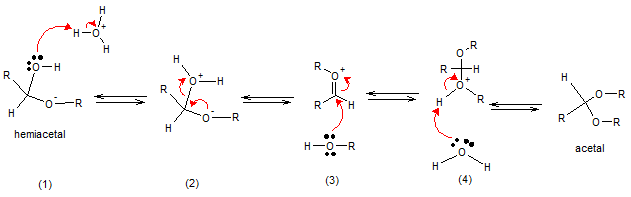
Mechanism for Acid-catalyzed acetal formation
An aldehyde or ketone in excess alcohol in the presence of an acid catalyst will form acetals. An acetal has two alkoxyl group on the same carbon. Formation of the acetal proceeds by forming hemiacetal intermediate, similar to hemiacetal formation where an acid catalyst protonates the carbonyl oxygen atom of aldehyde making the carbon in the carbonyl group more susceptible to nucleophilic addition of alcohol. Deprotonation with water will then yield hemiacetal.
In the presence of acid (NOT BASE), hemiacetals can undergo an elimination reaction. These are the stages of the mechanism. (1) Protonation of the hydroxyl group of the hemiacetal. (2) Loss of water by elimination. (3) This elimination leads to an unstable and highly reactive oxonium Ion and addition of methanol to the oxonium ion (4) loss of a proton to give the acetal.
Acetal formation from ketones and simple alcohols is less favorable than formation from aldehydes however, formation of 5- or 6- membered ring acetals from ketones is favorable. It is convenient to use a diol, to form a cyclic acetal because the reaction goes even more readily.

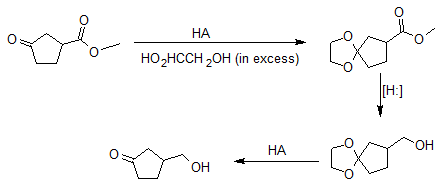
Furthermore, acetals can serve as protecting groups for aldehydes and ketones. Acetal protecting groups are stable to most reagents except aqueous acid. In the example below, reduction reaction of esters, cyclic acetals protects the carbonyl group from being reduced as well.