Keto-Enol Tautomerism
KETO-ENOL TAUTOMERISM
You may have first encountered keto-enol tautomerism when you were studying hydration of alkynes. In the said reaction, the alkyne is converted to a compound with a carbon-carbon double bond and one of the carbon in the C=C bearing a hydroxyl group (-OH). This particular structural feature is commonly known as an enol (ene + ol).
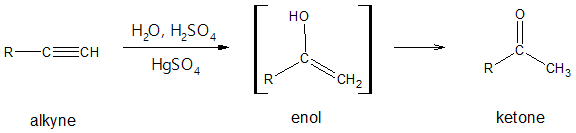
Surprisingly, the enol product was not isolated after the hydration of the alkyne. The predominant product turned out to be a ketone. From the above reaction, the enol served as an intermediate and quickly rearranges to a ketone through the process called keto-enol tautomerism. The keto and enol form are considered to be tautomers, the term used to describe constitutional isomers that interconvert rapidly. The keto and enol interconversion exist as an equilibrium process and to understand more about which of the two is predominant at equilibrium, we will use carbonyl combounds such as aldehydes and ketones in illustrating keto-enol tautomerism.
Equilibrium between Keto and Enol form
The two constitutional isomers keto and enol form are in equilibrium with each other.
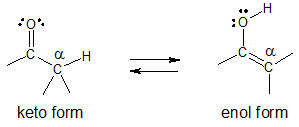
Comparing the two tautomers above, a keto tautomer has a C=O and an additional C–H bond while an enol tautomer has an O–H group bonded to a C═C.
As a review, equilibrium tells about the extent of a reaction, that is, which side of the reaction predominates when the concentration of reactants and products cease to change. In most cases, the keto form is favoured mainly because a C═O is much stronger than a C═C.
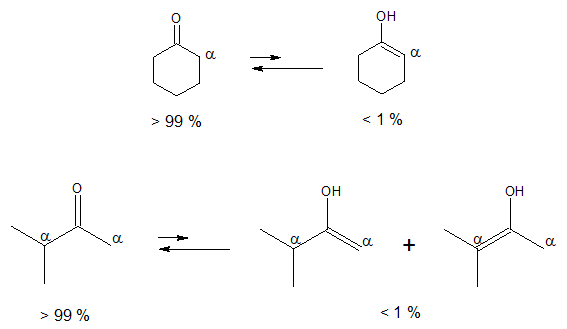
Both examples above illustrate the predominance of the keto tautomer over enol form. The question now is that is there a case where the enol form is favoured rather than the keto form? The answer is yes, especially in the case of compounds containing two carbonyl groups separated by a single carbon, also known as β-dicarbonyl compounds or 1,3-dicarbonyl compounds.
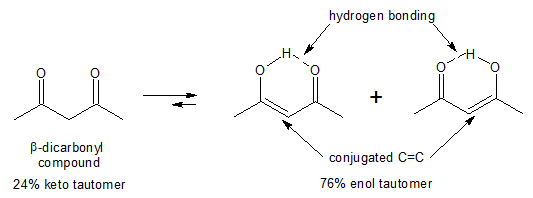
From the equilibrium interconversion shown above, it can be seen that conjugation and intramolecular hydrogen bonding are the factors that stabilizes the enol tautomer of β-dicarbonyl compounds. The C═C of the enol is conjugated with the carbonyl group, allowing delocalization of the electron density in the π bonds. In addition, the hydroxyl group (-OH) of the enol can hydrogen bond to the oxygen of the nearby carbonyl group. Such intramolecular H-bonding are especially stabilizing when they form a six-membered ring, as in the case above.
Based-Catalyzed Keto-Enol Tautomerism
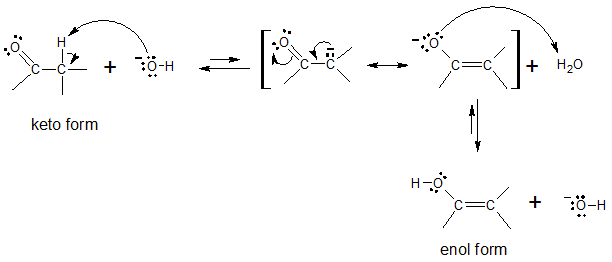
Notice that the above mechanism is composed of two major steps: (1) deprotonation of α carbon (α carbons are carbon right next to a carbonyl group, C═O; and (2) reprotonation on O in the intermediate formed. The hydroxide ion (OH-) serves as the catalyst in the process and is regenerated in the final step of the interconversion.
Acid-Catalyzed Keto-Enol Tautomerism
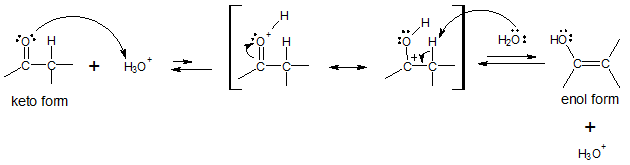
The hydronium ion (H3O+) representing the acid catalyzes the above tautomerism by protonating the O atom of the C=O group. The intermediate formed then undergoes deprotonation at the α carbon, resulting to the enol form. Look at the table to see the main difference between the two mechanisms presented.

Another consideration is that there must be a hydrogen atom on an α carbon that can be lost and regained through keto-enol tautomerism and the hydrogen is said to enolizable.
