Synthesis of Acyl Halides
Synthesis of Acyl Halides
Closely related to carboxylic acids are the carboxylic derivatives, compounds in which an acyl group is bonded to an electronegative atom or substituent that can act as a leaving group in a substitution reaction. There are many known acid derivatives, one of which is the acyl halides or acid halides. Acid halides are derivatives that are mostly used in the laboratory unlike other acid derivatives which are also found in biological systems.
Speaking of laboratory, at this point we will explore the common methods on how acyl halides are synthesized or derived from carboxylic acids. Basically we will answer the question mark below.
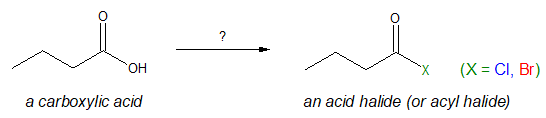
The conversion of a carboxylic acid to an acid derivative such as acyl halide is known as nucleophilic acyl substitution. At a glance, it would look like we are simply replacing the –OH group of the starting carboxylic acid with a halide atom (-X). But just like in alcohol reactions, you’ve learned that –OH group is not a good leaving group for a substitution reaction. Nevertheless, under right circumstances, acyl halides can still be prepared from carboxylic acids.
Acyl Chloride from Carboxylic acid
Commonly encountered acyl halides in organic chemistry are acyl chlorides so we will focus on producing acyl chlorides from carboxylic acids. Most common reagent used to carry out the said conversion is through the use of thionyl chloride, SOCl2 in organic solvent such as chloroform, CHCl3.
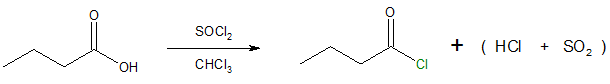
To better understand the above transformation, look at the mechanism that follows. To sum it up, the reaction occurs by a nucleophilic acyl substitution pathway in which the carboxylic acid is first converted as a better leaving group in the form of a chlorosulfite intermediate (see the mechanism). A chloride ion (Cl-) then acts as the nucleophile in the process.
Mechanism of converting carboxylic acid (–COOH) to acyl chloride (–COCl):
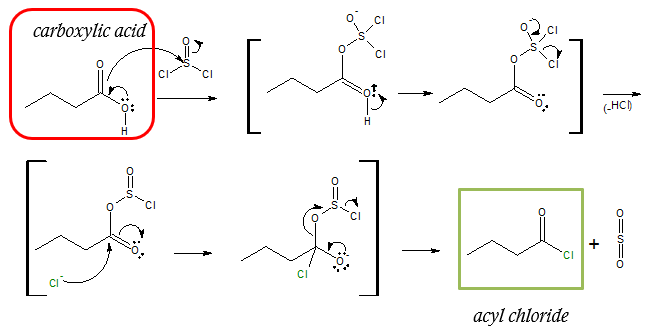
Take note that the nucleophilic acyl substitution occurs on the –COOH portion of the molecule, converting it to –COCl group. So regardless of the starting carboxylic acid, preparation of acyl chlorides via SOCl2 is basically replacing the OH group to –Cl atom.
Acyl Bromide from Carboxylic acid
Other acyl halides commonly encountered in organic synthesis are acyl bromides which can be synthesized starting from a carboxylic acid with the use of PBr3 in ether. Just like in the formation of acyl chlorides, the utilization of PBr3 replaces the –OH group of the carboxylic acid functional group with a –Br atom via nucleophilic acyl substitution. Look at the example below.
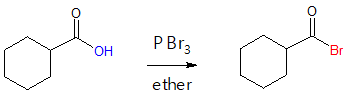
The mechanism for the above transformation is quite similar with that of the synthesis of acyl chloride from carboxylic acid.
