Conversion to Anhydrides
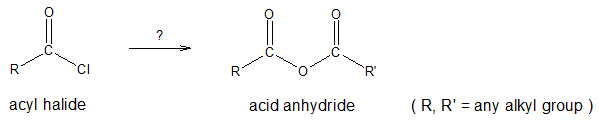
CONVERSION OF ACYL HALIDES TO ANHYDRIDES
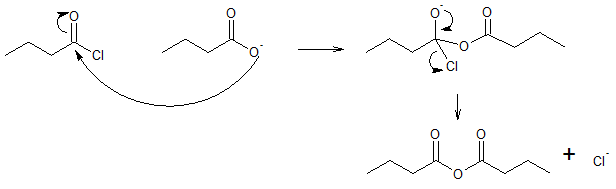
Acid halides are considered as the most reactive among the carboxylic acid derivatives and thus can be converted into many other kinds of compounds by nucleophilic acyl substitution. In this article, we will try to look into the reaction of acyl halides to form acid anhydrides.
Like any acyl-containing compound, the above conversion can be accomplished allowing the acyl halide to react with salt of a carboxylate ion. As a review, a carboxylate ion results when the acidic hydrogen of the carboxylic acid compound is removed by a base. Example is shown below:
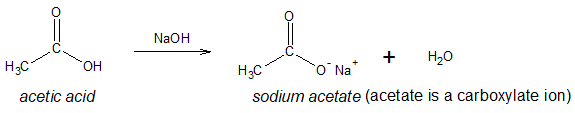
Like any acyl-containing compound, the above conversion can be accomplished allowing the acyl halide to react with salt of a carboxylate ion. As a review, a carboxylate ion results when the acidic hydrogen of the carboxylic acid compound is removed by a base. Example is shown below:
Symmetrical anhydride:
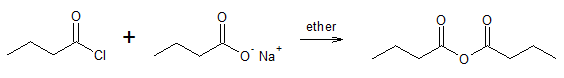
Unsymmetrical anhydride:
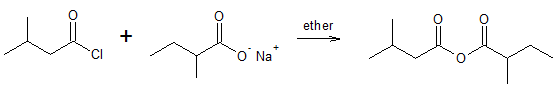
Look at the mechanism that follows.
Mechanism for nucleophilic acyl substitution between acyl halide and carboxylate ion: