Conversion of Acyl Halides to Carboxylic Acids
CONVERSION OF ACYL HALIDES TO CARBOXYLIC ACIDS
Maybe you’ve already learned about converting carboxylic acids to acyl halides in previous articles. In fact, acyl halides are the most reactive of all the carboxylic acid derivatives and due to their sufficient reactivity, acid halides can easily be converted back to carboxylic acids with reaction with water (H2O). This specific reaction will be the meat of this article, also known as hydrolysis reaction of acyl halides to form carboxylic acids.
The hydrolysis reaction of acyl halides such as acyl chlorides is a typical nucleophilic acyl substitution process to yield carboxylic acids. This reaction is initiated by attack of the oxygen (O) atom of water on the acid chloride carbonyl group. Water serves as the nucleophile while the carbonyl carbon acts as the electrophilic site. An example is shown below.
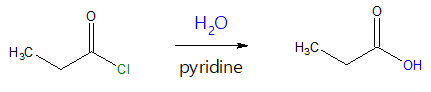
If you follow the pattern of the above reaction, the hydrolysis reaction of acyl chloride reactant can be viewed simply as replacement of the chloride (-Cl) group (or halide group in general) with a hydroxyl group (-OH). Look at the mechanism below for a clearer picture of the said transformation.
Mechanism:
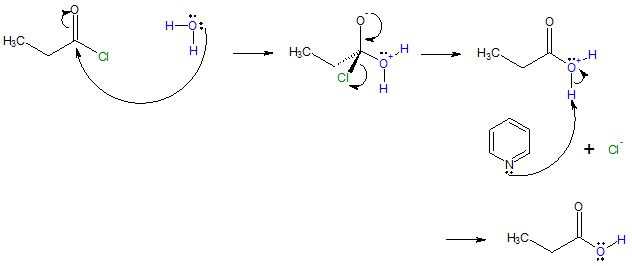
Because HCl is a by-product of the reaction, the hydrolysis is usually carried out in the presence of a base such as NaOH or in the above case, pyridine, to remove the HCl and prevent it from causing side reactions.
More examples are illustrated below:
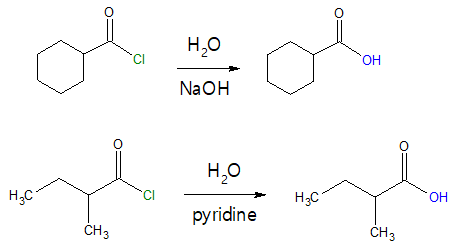
Always remember that hydrolysis (reaction with H2O) of acyl chloride yields a carboxylic acid to which the said acid halide was derived. Overall, you are just simply replacing the –Cl group with an –OH group.
