Curtius Rearrangement
CURTIUS REARRANGEMENT
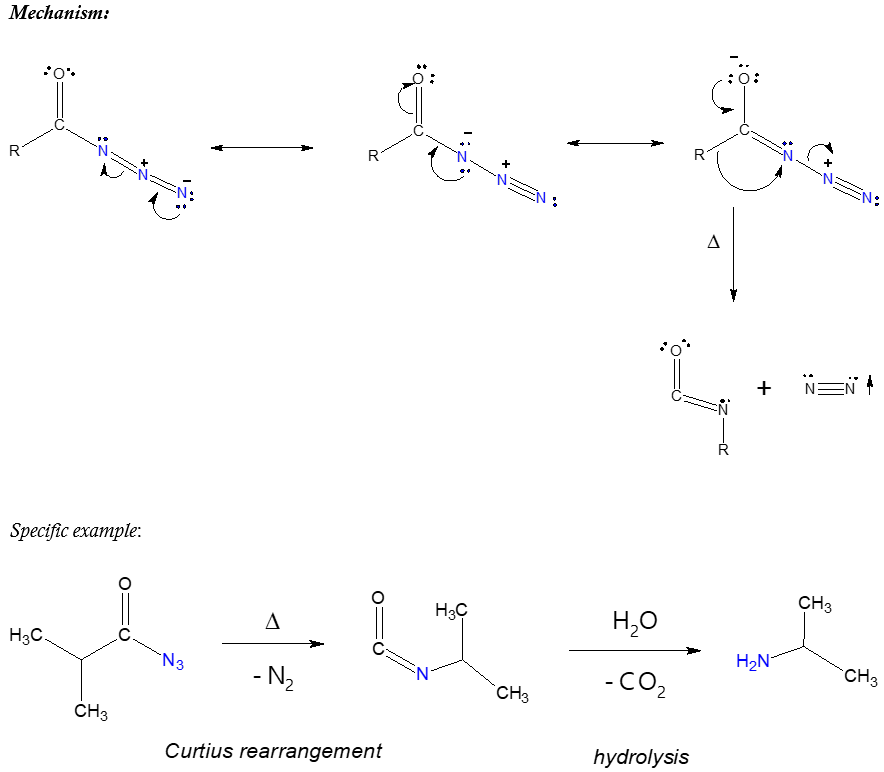
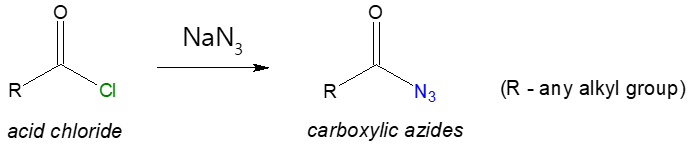
Carboxylic acid could also be modified to introduce a nitrogen group in the molecule. This acid derivative is commonly known as carboxylic azide or acyl azide. Recall that acid chlorides are relatively more reactive than carboxylic acids, thus, if you want to prepare acyl azides, the most practical way is to start with acyl chloride and allow it to react with sodium azide (NaN3). Such reaction pathway is shown below.
You may wonder why we are talking about carboxylic azides. Simply because when we speak of Curtius rearrangement reaction, acyl azide is the starting chemical reagent. This particular reaction involves the thermal decomposition of acyl azides to yield isocyanate. When we say thermal decomposition, applying heat would make the rearrangement possible such as illustrated below.
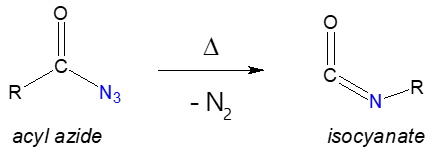
What could be the possible of the isocyanate produced? Isocyanate can potentially proceed to a further reaction in the presence of nucleophiles (electron-rich species) in solution. The said reaction intermediate can be isolated if you will or it can undergo hydrolysis reaction wherein the products may be obtained if desired. The hydrolysis of isocyanate yields amines which tells us that if one desires to synthesize an amine, performing Curtius rearrangement starting from an appropriate acyl azide followed by hydrolysis is a possible option or pathway. The isocyanate product of Curtius reaction is considered a versatile compound that can be utilized to synthesize a number of nitrogen-containing compounds.