Conversion of Acyl Halides to Amides
CONVERSION OF ACYL HALIDES TO AMIDES
You may have encountered a lot of transformations that acid halides could undergo and mostly oxygen-containing group replaces the halide group. Due to characteristic high reactivity of acyl halides, it is also possible to introduce a nitrogen-containing group still via nucleophilic acyl substitution.
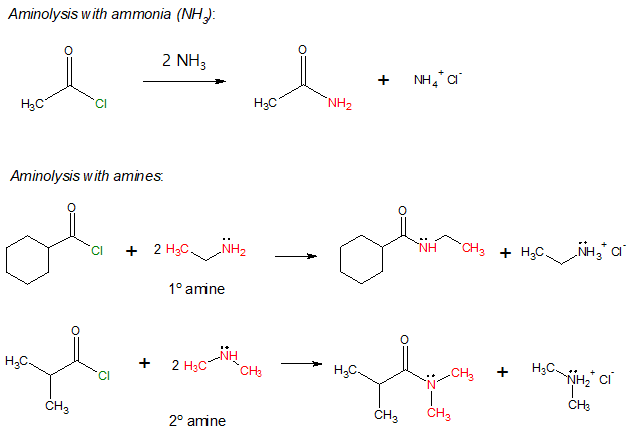
Notice from the above examples that normally, two equivalents of ammonia or amine are needed in carrying out the reaction. This is because like any other nucleophilic acyl substitution with acyl chlroides, HCl is produced. One mole of ammonia or amine acts as the nucleophile while the second mole serves reacts with HCl to form a chloride salt.
It is important to consider the degree of substitution of the amine involved in the reaction. Referring to the examples, only primary or monosubstituted (1o) and secondary or disusbtituted (2o) amines were used indicating that tertiary or trisubstituted (3o) amines do not react with acyl chlorides to produce amides.
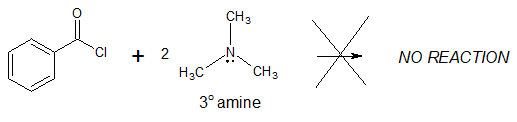
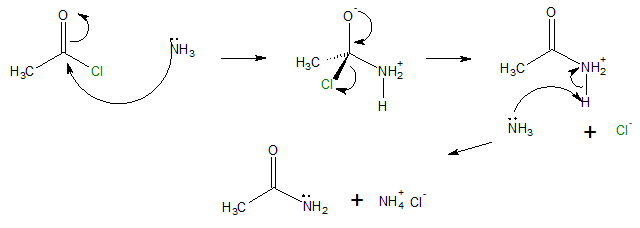
Therefore, in the conversion of acyl chlorides to amides, two equivalents of either ammonia, primary or secondary amines can be used through nucleophilic acyl substitution mechanism.
