Conversion of Acyl Halides to Esters
CONVERSION OF ACYL HALIDES TO ESTERS
Due to high reactivity of acyl chlorides compared to other carboxylic acid derivatives, converting acyl chlorides into other acid derivatives. At this point, we will try to understand how an acyl halide is converted to an ester. Maybe you already have an idea about converting acyl halides to acid anhydrides. Fortunately, the same mechanism is involved and that is still a nucleophilic acyl substitution reaction.
To convert an acyl halide to an ester, an alcohol reactant is required. This is the reason why the conversion is also referred to as alcoholysis. An example is shown below.
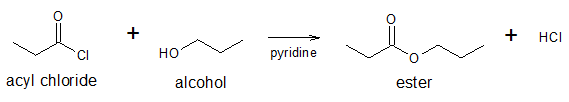
As mentioned earlier, the mechanism is nucleophilic acyl substitution wherein the C atom of the C=O in –COCl group acts as the electrohile and the O atom of the alcohol acts as the nucleophile. The process is analogous to the reaction of acyl chloride with water to yield carboxylic acid. The reaction mechanism is shown below.
Mechanism:
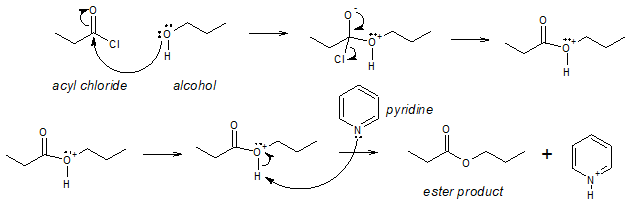
As with hydrolysis, alcoholysis reactions are usually carried out in the presence of pyridine or NaOH to react with the HCl formed.
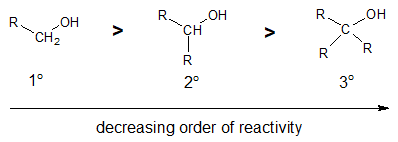
The reaction of an acyl chloride with an alcohol is greatly affected by steric effect. Bulky groups on either the acyl chloride or alcohol tends to slow down the reaction to a considerable extent. Hence, the order of reactivity among the different degrees of alcohol toward nucleophilic substitution with acyl chloride would be:
In addition, the steric hindrance factor also gives the alcoholysis of acyl chloride some selectivity. That is, it is possible to produce an ester (often called to esterify) from an unhindered alcohol selectively in the presence of a more hindered one. This can be important especially in complex syntheses and distinguishing between similar functional groups present in the same molecule is desired. To appreciate this more, look at the example below.
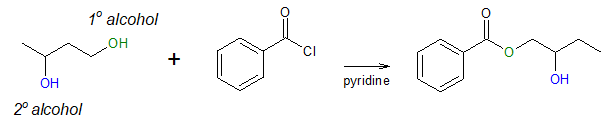
Notice that in the above example, the alcohol molecules contains two –OH group, one is a primary (1o) alcohol (the green –OH) and the other is a secondary (2o) alcohol (the blue –OH). As mentioned earlier, a less sterically hindered 1o alcohol is more reactive than a more hindered secondary alcohol. As a result, esterification occurs at the 1o –OH, not on the 2o –OH.
