Conversion of Acyl Halides to Aldehyde and Ketones
Conversion of Acyl Halides to Aldehyde and Ketones
The high reactivity of acyl chloride relative to other carboxylic acid derivatives could also be utilized to prepare other important acyl compounds. At this point, we will consider the preparation of aldehydes and ketones from acyl chlorides.
Reduction of Acyl Chlorides into Aldehydes
If you want to produce aldehydes from acyl chloride, the best method available thus far is to use an appropriate reducing agent. Maybe you are already familiar with lithium aluminium hydride (LiAlH4) as a strong reducing agent. By being strong means that the reduction of one compound to another is difficult to control especially if you have a particular product that you want to produce. As an example, acyl chlorides are reduced all the way to a primary alcohol as shown below: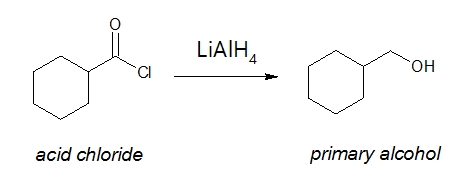
The question now is how can one control the reduction process so that one just ends up with an aldehyde compound? Fortunately, there exists a milder reducing agent known as lithium tri-tert-butoxyaluminum hydride [Li+ -AlH(O-t-Bu)3], LATB-H. It is considered milder than LiAlH4 in the sense that it reacts faster with acid chlorides than with aldehydes. Hence, the competing reduction of acid chlorides to aldehydes (faster) and aldehydes to alcohols (slower) makes it possible to isolate the aldehyde formed in the process.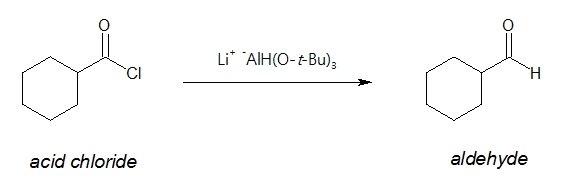
The mechanism for acid chloride reduction proceeds via a hydride (H-) transfer from the reducing agent to the acid chloride. The steps involved are summarized in the figure that follows.
Mechanism:
1. An acid-base reaction occurs between an electron pair on the oxygen of the carbonyl group and the aluminium atom of the LATB-H.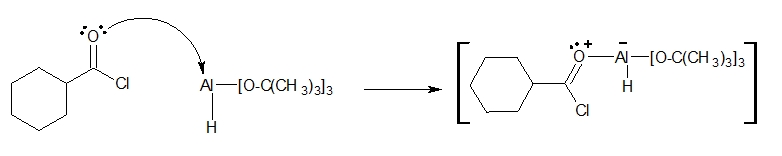
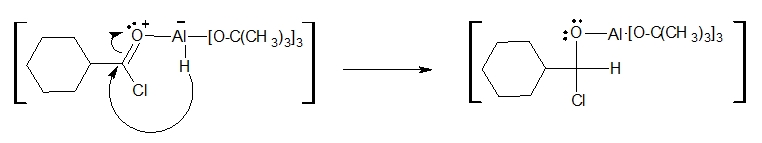
3. A chloride ion (Cl-) is released when one of the lone pairs of the carbonyl oxygen delocalizes back to reform the C=O bond.
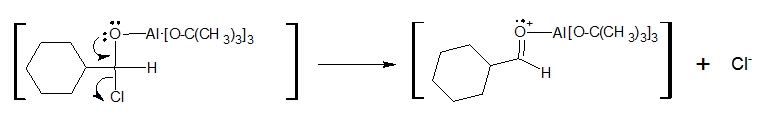
4. Finally, the aluminium complex is taken out of the picture by hydrolysis upon addition of water to produce the aldehyde
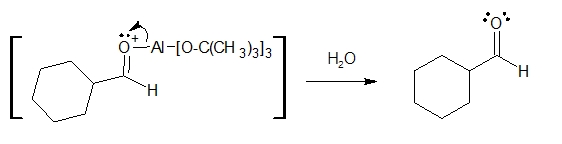
As you can see, the overall reaction is basically replacing the Cl atom of the acid chloride with an H atom (came from LATB-H as hydride ion) to form the corresponding aldehyde compound.
Synthesis of Ketones from Acid Chlorides
Ketones which are closely related to aldehydes can also be made out of acyl chlorides. This time, instead of a hydride-containing reagent, a chemical reagent with R- (a negatively charged alkyl group or also called a carbanion) is preferably used. Grignard and organolithium reagents are among the compounds containing an R- commonly used in organic synthesis.
A reaction involving Grignard reagent is shown below.
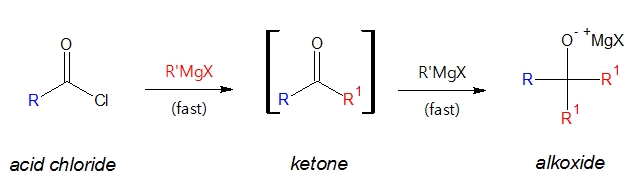
Notice that the formation of a ketone from an acid chloride using a Grignard reagent was partially achieved, partial in a sense that the initially produced ketone readily reacts with another mole of Grignard reagent forming a tertiary alkoxide ion.
To prevent the undesirable formation of alkoxide product and to limit the reaction only up to the ketone stage, a weaker organometallic reagent is used, one that reacts faster with acyl chlorides than with ketones. In this case, the alkoxide formation would be minimized and the ketone compound is predominantly formed. One of the organocuprates that can be utilized for the said purpose is the lithium dialkylcuprates. Look at the reaction below on how lithium dialkylcuprate is formed.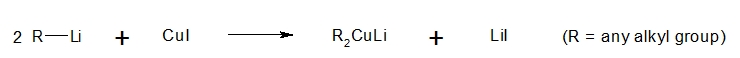
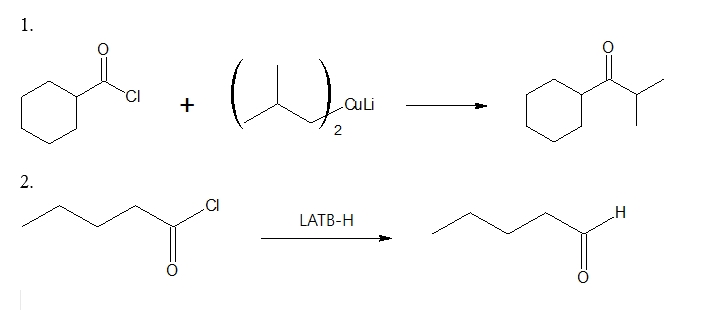
After synthesizing the necessary lithium dialkylcuprate, it is then allowed to react with an appropriate acid chloride to form the desired ketone. Specific example is shown below.
Lithium dialkylcuprate formation: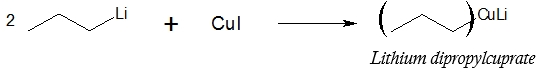
Ketone formation: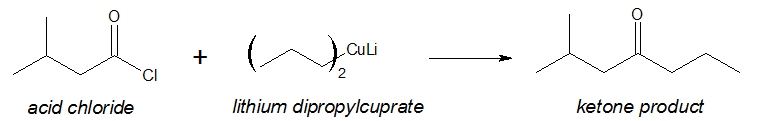
The mechanism for the ketone formation starting with acid chloride is very similar to the aldehyde synthesis from acid chloride. The only difference is that carbanion is involved instead of a hydride ion.
Further examples of aldehyde and ketone synthesis from acid chlorides: