Ring Reduction
Ring Reduction
Ring reduction or ring contraction is a type of organic reaction in which the size of the ring is decreased. The initial substrate after ring contraction produces exocyclic carbon. Following scheme depicts the general reaction of ring contraction.
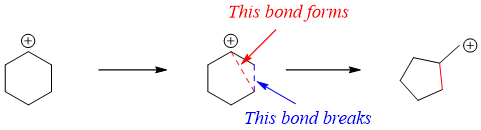
Following are some example reactions including ring contraction rearrangements.
FAVORSKII REARRANGEMENT:
The Favorskii rearrangement is the reaction involving conversion of α-haloketones into carboxylic acid derivatives in basic medium. This rearrangement is mainly used for the synthesis of highly branched organic systems. In this reaction the α-haloketone is reacted with base to generate corresponding enolate. The enolates undergo intramolecular reaction and eliminates halogen atom to form cyclopropanone intermediate.
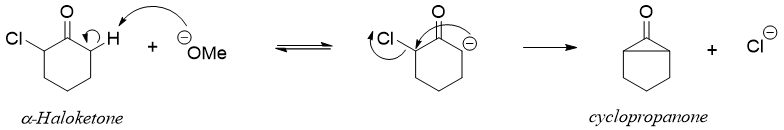
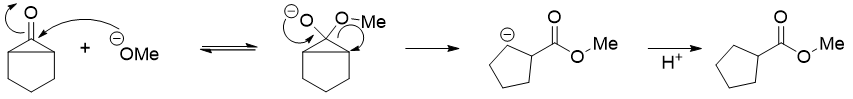
The cyclopropenone intermediate cleaves to form the most stable carbanion.
WOLFF REARRANGEMENT:
In Wolff rearrangement reaction ring contraction is performed by first transforming cyclic α-diazoketones into ketenes. Ketenes are then reacted with different nucleophiles to produce different products.
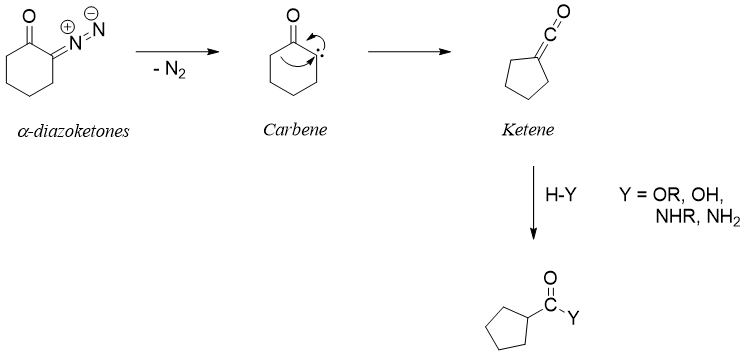
The formation of carbene from α-diazoketone is initiated by different means including heat, light or using transition metals to evolve nitrogen gas.
RING CONTRACTION THROUGH CATIONIC INTERMEDIATE:
Ring contraction reactions can also be achieved by utilizing cationic intermediate. For example, the rearrangement of epoxides in the presence of Lewis acid using non-nucleophilic solvents.
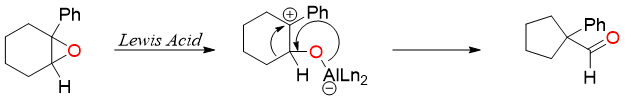
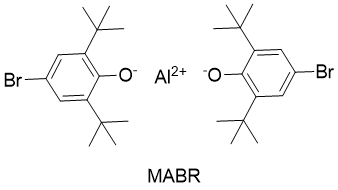
The Lewis acids used in such reactions are bulky in nature. One common Lewis acid used for the ring contraction reactions is methylaluminum Bis(4-bromo-2,6-di-tert-butylphenoxide) (MABR).
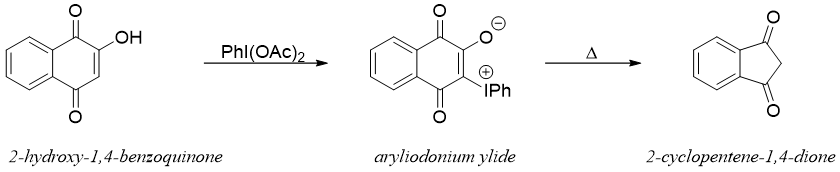
THERMOLYSIS OF ARYLIODONIUM YLIDES:
In this method 2-hydroxy-1,4-benzoquinones are transformed into 2-cyclopentene-1,4-diones with a reduction in ring size by one carbon. In this method the 2-hydroxy-1,4-benzoquinones are reacted with hypervalent iodine reagent like diacetoxy iodobenzene (PhI(OAc)2) to produce phenyl iodonium (an aryliodonium ylide) which upon thermolysis produces corresponding 2-cyclopentene-1,4-diones.