Beckmann Rearrangement
Beckmann Rearrangement
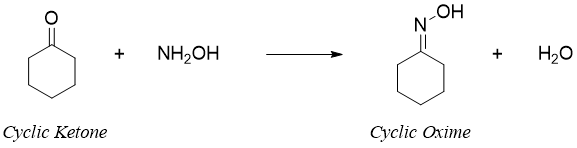
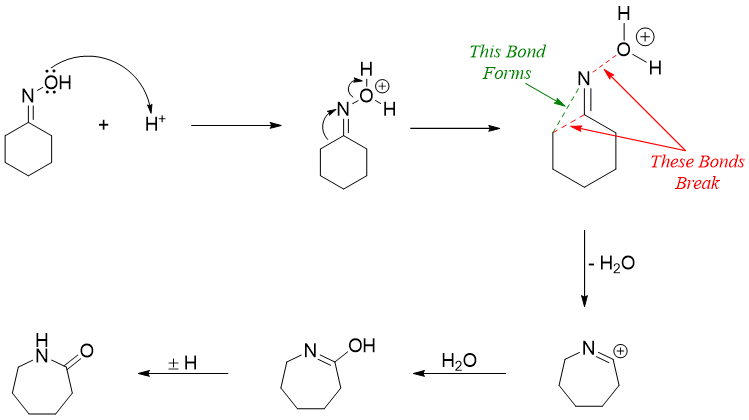
Beckmann rearrangement is an acid catalyzed transformation of an oxime to an amide. In this rearrangement cyclic ketones are converted into lactams. The mechanism of Beckmann rearrangement follows the same pattern as a pinacol rearrangement in which the -OH group of oxime is converted into a good leaving group by protonating it with an acid. The water group eliminates with the migration of the alkyl group. The final product cation reacts with water to generate an amide functional group.
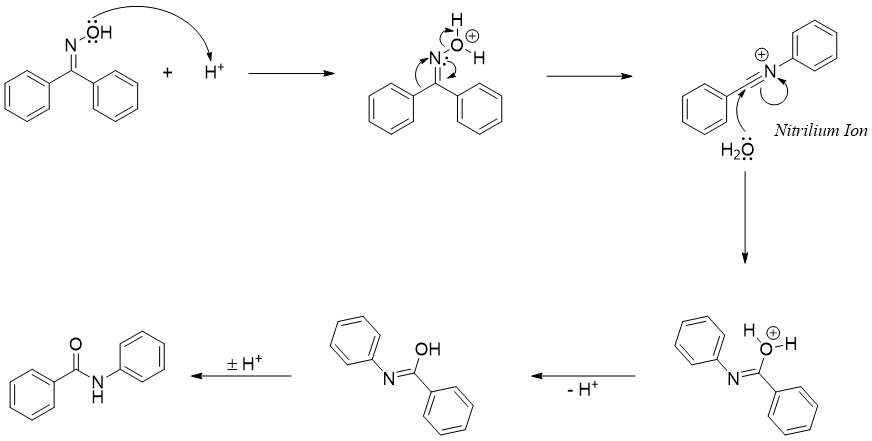
Beckmann rearrangement is not restricted to cyclic oximes. In acyclic Beckmann rearrangement, the final product is represented as nitrilium ion which shows the involvement of lone pair of electrons of nitrogen atom in pushing the migrating group.
In Beckmann rearrangement of unsymmetrical ketones there are two different groups that can migrate. Furthermore, the unsymmetrical oxime can have two different geometrical isomers i.e., cis and trans. In Beckmann rearrangement, the group that migrates is trans to the leaving group. For example, the oxime of 2-pentanone has a mixture of geometrical isomers. After rearrangement the ratio of products (73:27) reflects exactly the ratio of geometrical isomers (75:25). The rearrangement works only with trans isomers because the migrating group has to interact with the σ* of the bond to the leaving group.
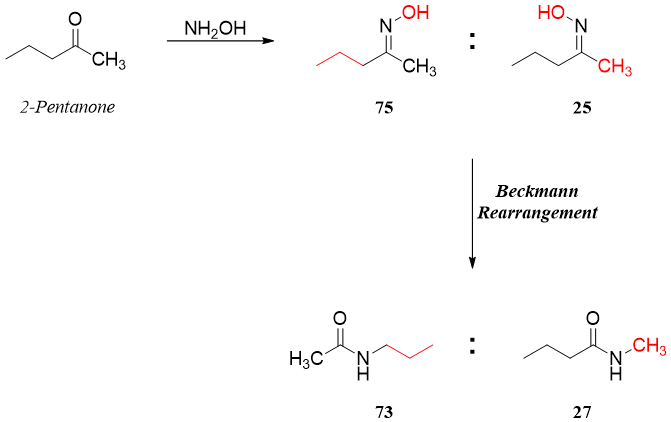
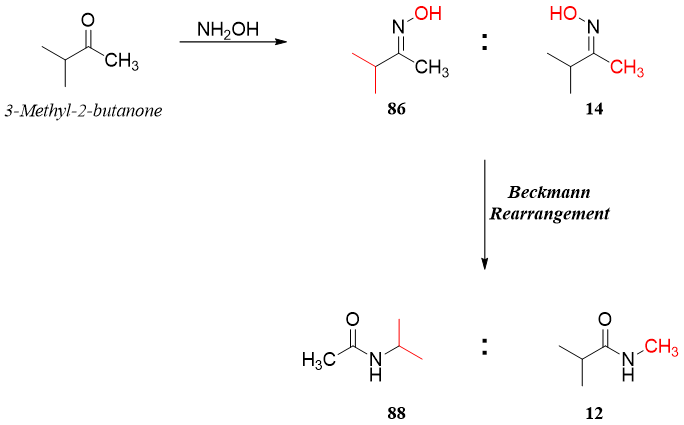
If the starting unsymmetrical ketone has branched alkyl chain, then the resulting oxime will form with the -OH anti to the branched chain with high ratio. Hence, in rearrangement reaction more branched alkyl chain will migrate.
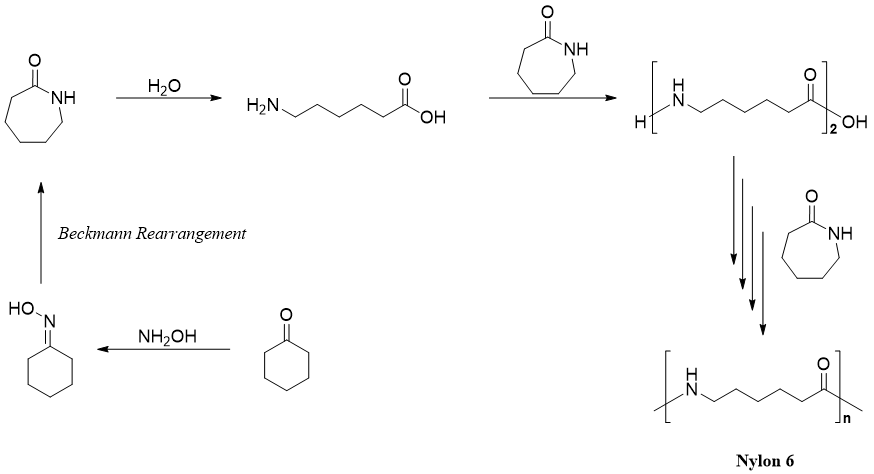
Beckmann rearrangement has many applications including synthesis of monomer used for the manufacture of Nylon 6.
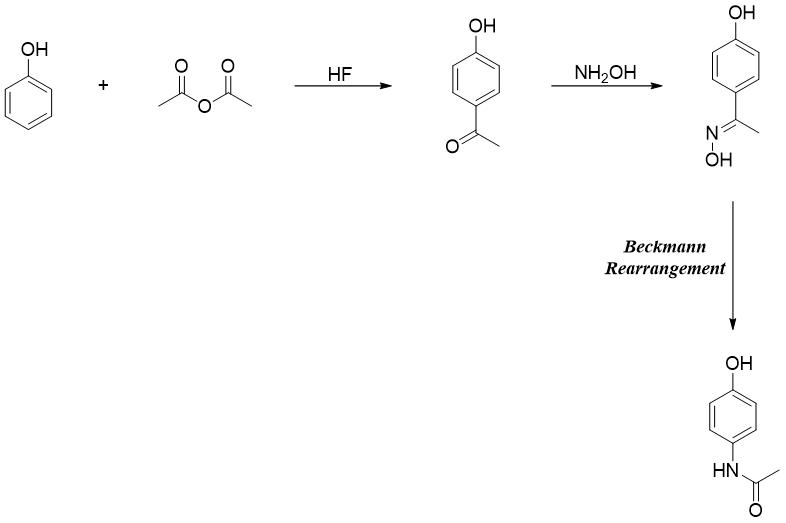
A common pain killer Paracetamol is also synthesized by Beckmann rearrangement reaction.