Hydride Shift
Hydride Shift
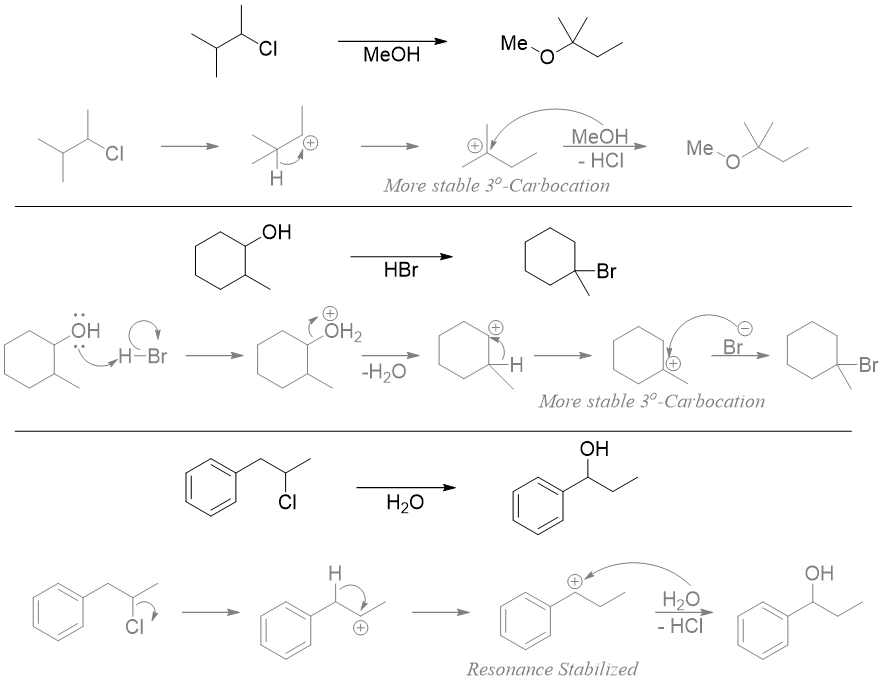
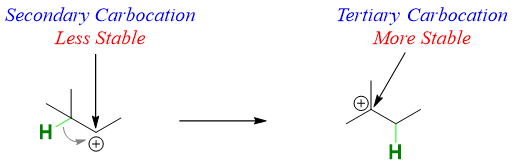
Hydride shift is a type of rearrangement reaction in which a hydrogen atom in a carbocation rearranges (shifts) its position to change that carbocation to more stable carbocation. The shifting of hydrogen from one carbon to another carbon results in a structural isomerism. As shown below, the C-H bond adjacent to carbocation interacts with the empty p orbital of carbocation and the bonding electron pair moves to form a stable tertiary carbocation.
The hydride shifts occurs when a substrate is changed into a carbocation. This can occur in different ways including SN1 reactions.
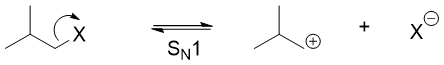
Once the carbocation is formed it will rearrange to change into more stable carbocation. In above SN1 reaction the carbocation is primary carbocation. Thus, the hydrogen at adjacent carbon will underdo 1,2-shift to form tertiary carbocation which is more stable.

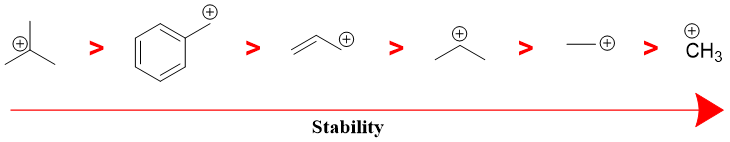
The order of stability of different carbocations is shown below.
Wagner-Meerwein Rearrangement:
It is a type of 1,2 rearrangement reaction in which hydrogen, alkyl or aryl group moves from one carbon to adjacent carbon. This reaction is also called 1,2-sigmatropic rearrangement reaction.
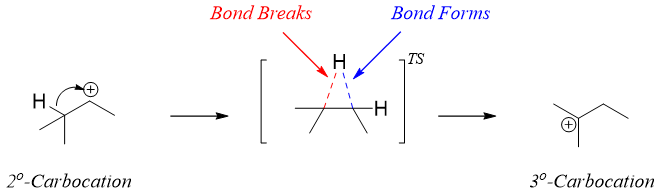
The hydride shifts only occurs when there is the possibility of formation of more stable carbocation. Hence, the driving force for the hydride shift is the stability of carbocation.
In following example, the hydride shift will result in the formation of same secondary carbocation. In this case the benzylic carbocation is formed because of its stability due to resonance.
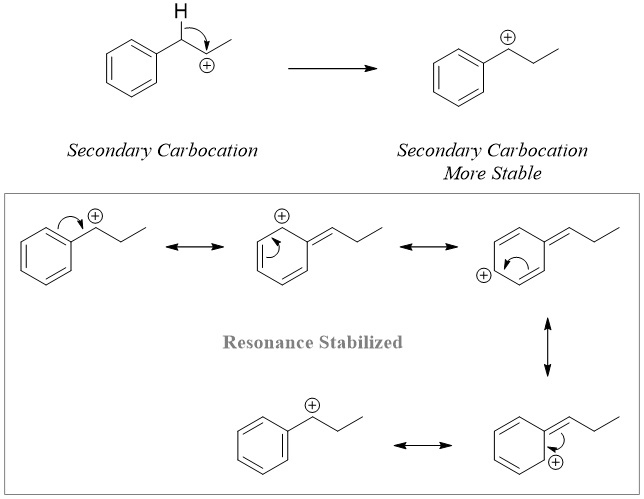
The hydride shift rearrangement mainly occurs in SN1 reactions. The carbocations formed in first step of SN1 reactions rearranges to form mores stable cation. After rearrangement the nucleophile adds to the carbocation. Following are some examples of SN1 reaction undergoing hydride shift rearrangement reactions.