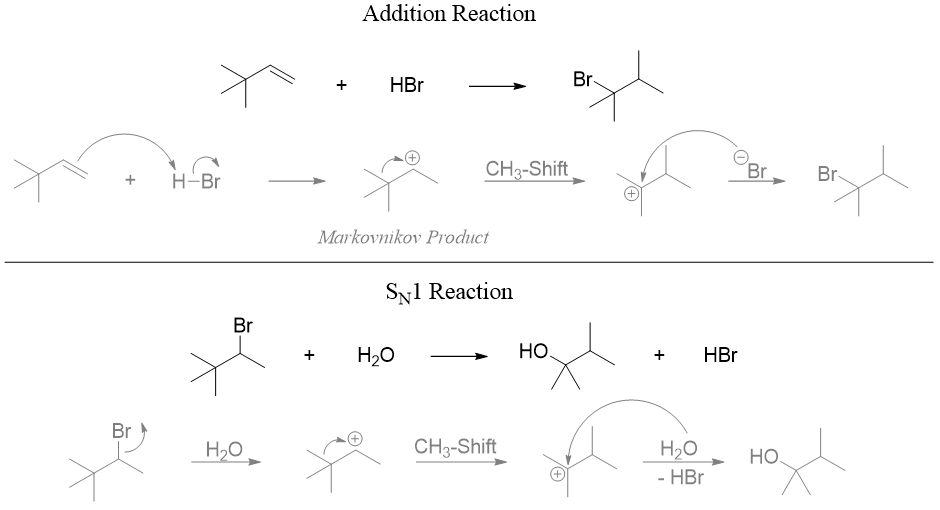
Methyl Shift
Methyl Shift
The carbocation can rearrange to change into more stable form. As discussed earlier, this rearrangement can take place by different ways including hydride shift. Sometimes there is a situation where the hydride shift would not produce a more stable carbocation, but a methyl shift would. For example, the SN1 reaction of 3-bromo-2,2-dimethylbutane will produce secondary carbocation. There are two possibilities for this carbocation to rearrange. 1) The 1,2-hydride shift will change this secondary carbocation to primary carbocation, 2) The 1,2-methyl shift will change secondary carbocation into tertiary carbocation. Since, tertiary carbocation is more stable then primary and secondary carbocation therefore, only methyl shift will take place.

Hence, methyl shift usually takes place in substrates in which a quaternary carbon is attached to secondary carbon containing leaving group.
The methyl shifting reaction works when the empty p orbital of carbocation aligns with the electron pair of C-CH3 bond in same plane. Once the bonded pair of electrons are donated the breakdown and formation of new bond takes place simultaneously.
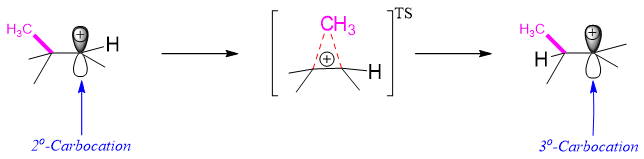
Following are some reactions involving methyl shift rearrangement reactions.