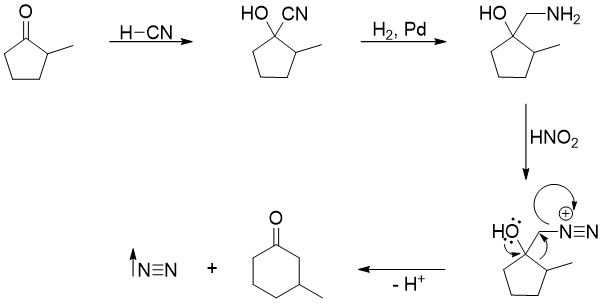
Ring Expansion
Ring Expansion
Rearrangement reactions take place whenever there is any possibility of formation of more stable intermediate during a chemical reaction. As discussed in earlier articles, hydride shift and methyl shift rearrangements take place to produce more stable carbocations. More stable carbocations mean more substituted carbocations. Stability can also be induced by relieving ring strains. For example, the ring strain in four membered ring is greater than the ring strain in five membered ring. The bond angle in four membered ring is 88° whereas, in cyclopentane the bond angle between C-C-C is 109.5°. Hence, if the four membered ring is adjacent to a carbocation, then the intermediate will readily rearrange to form five membered ring to relieve the ring strain.
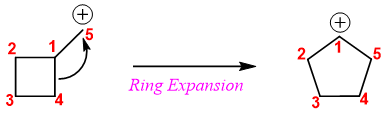
In above example, two factors are favoring the expansion of ring. 1) Relieve in ring strain from 4-membered ring to 5-membered ring, 2) conversion of primary carbocation into secondary carbocation.
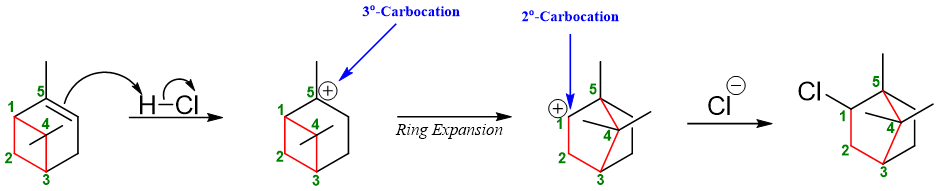
In some cases, the relieve in ring strain dominates over carbocation stability. For example, in following example, highly stable tertiary carbocation is changed to less stable secondary carbocation but still the rearrangement occurs as the relieve in ring strain makes the intermediate more stable.
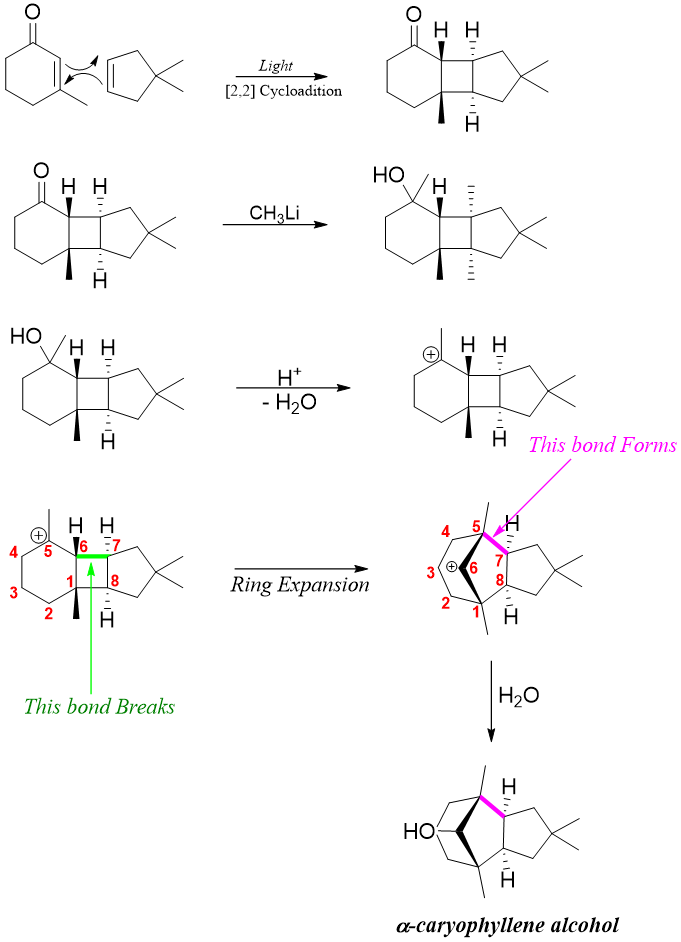
Another example of ring expansion includes the formation of natural product α-caryophyllene alcohol.
Following are some methods/reactions involving ring expansion rearrangements.
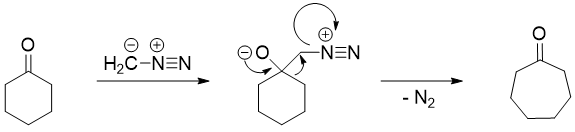
Wolf Rearrangement:
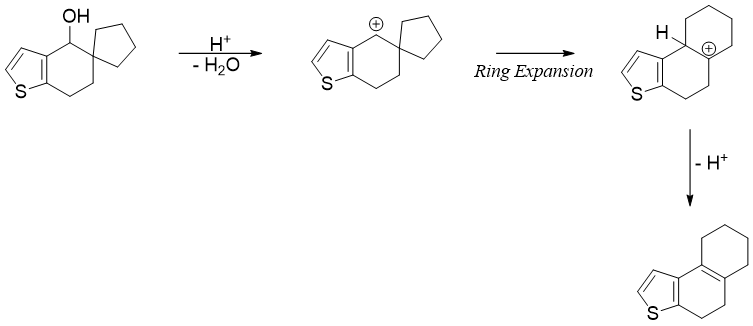
Wagner- Meerwein rearrangement:
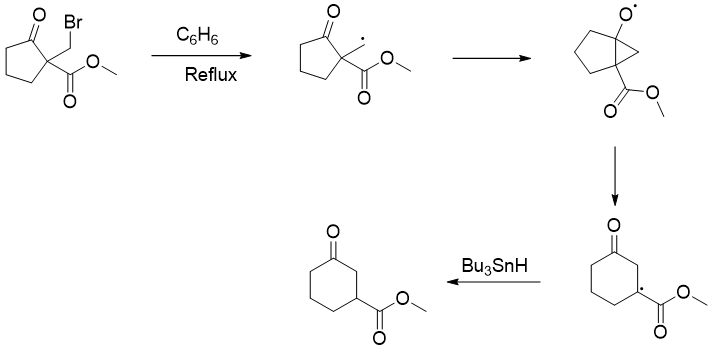
Dowd-Beckwith ring Expansion:
Tiffeneau-Demjanov rearrangement: