Pinacol Rearrangement
Pinacol Rearrangement
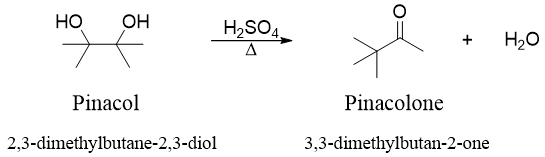
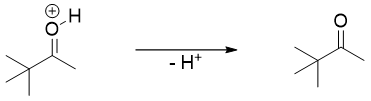
Diols undergo a unique reaction when treated with acid. This reaction is the dehydration reaction with a strange rearrangement. The dehydration of diol shown below is an example of pinacol rearrangement.
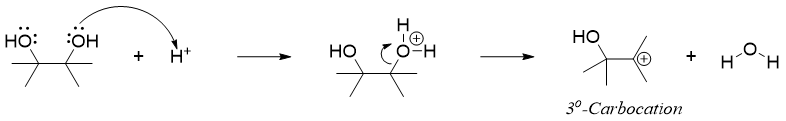
The mechanism of pinacol rearrangement initiates with the protonation of one of the hydroxyl oxygen atoms. Protonation converts -OH to a good leaving group i.e., H2O. The loss of water molecule forms a tertiary carbocation.
Once the tertiary carbocation is formed, the methyl group on adjacent carbon to carbocation migrates to carbocation to form resonance stabilized carbocation.
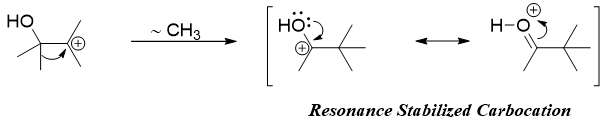
The stability provided to carbocation by the resonance of non-bonded electrons of oxygen atom is the driving force of pinacol rearrangement reaction. The deprotonation of the resonance stabilized carbocation yields the final product.
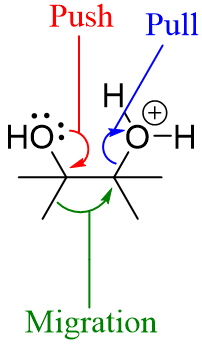
The pinacol rearrangement mainly works on “push” and “pull” mechanism. The water molecule leaving the molecule pulls the migrating group while, the non-bonded electrons on oxygen atom pushes the migrating group.
Pinacol rearrangement reactions have many applications including synthesis of spirocyclic ring systems. For example.

The carbocation intermediate formed in pinacol rearrangement can also be formed by other means. The opening of epoxide ring by treating epoxide with acid including Lewis acids can undergo pinacol rearrangement.
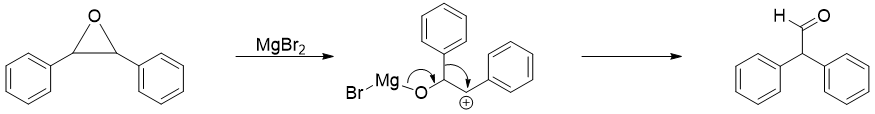
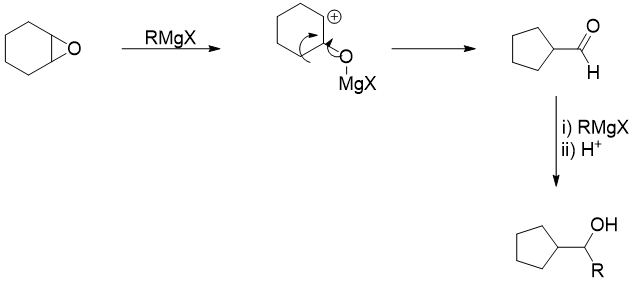
From above example it is concluded that the reaction of epoxides with magnesium salts like Grignard reagent can give surprising products. For example, in following reaction when epoxide is reacted with Grignard reagent the rearrangement reaction take place first. This step is the fastest step and converts the epoxide to an aldehyde. In the second step, the aldehyde reacts with Grignard reagent to produce corresponding alcohol.
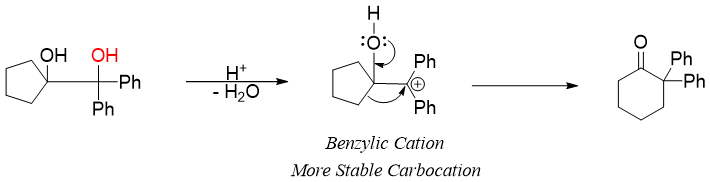
In case of unsymmetrical diols, the hydroxyl group which upon protonation and elimination produces more stable carbocation is eliminated. For example, in following starting molecule the elimination of red -OH group will produce more stable carbocation as the two phenyl groups stabilizes the carbocation by resonance.