Curtius Rearrangement
Curtius Rearrangement
In Curtius rearrangement carboxylic acid derivatives are transformed into primary amines with the loss of one carbon atom. The reaction starts with acyl azide, which is formed by reaction of acyl chloride and sodium azide. Upon heating, the acyl azide decomposes to produce nitrogen gas and nitrene.
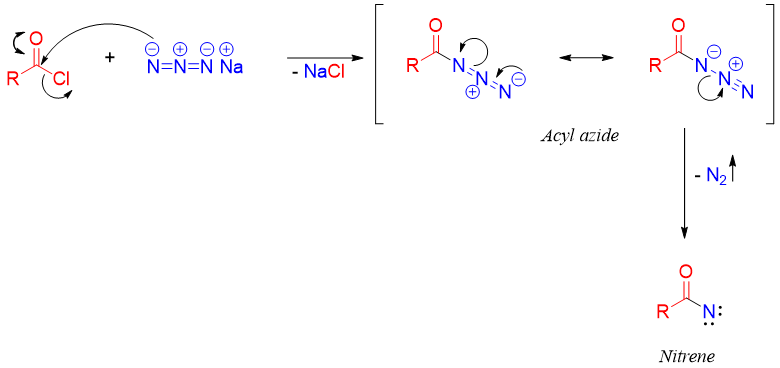
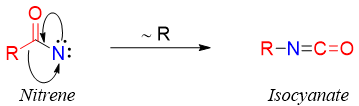
Nitrenes are extremely reactive and electrophilic in nature. Migration of R group takes place and moves to the electron deficient nitrogen atom of nitrene forming isocyanate.
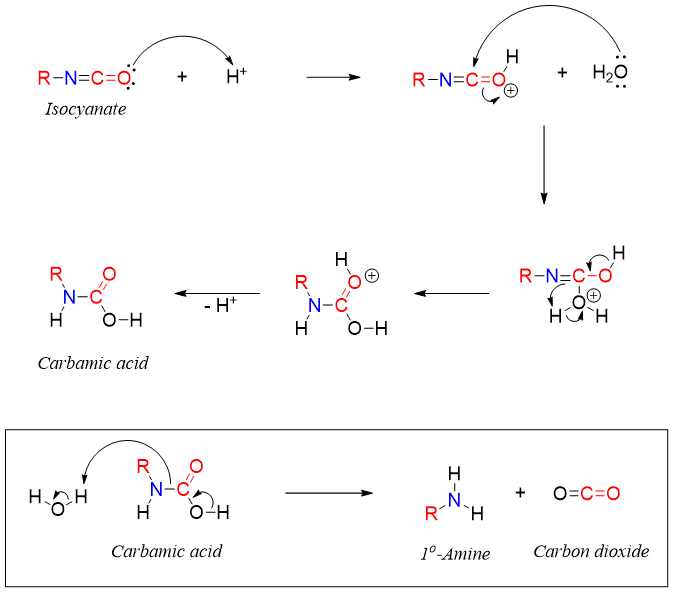
Isocyanate upon hydrolysis produces carbamic acid which further decomposes to primary amines.
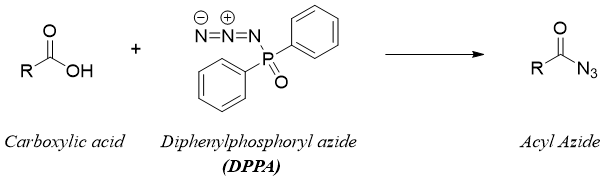
The Curtius rearrangement can be started directly from carboxylic acid. The carboxylic acid upon reaction with diphenylphosphoryl azide (DPPA) produces corresponding acyl azide derivative.
Curtius rearrangement reaction has many applications including synthesis of different functional groups. The isocyanate formed when reacted with different nucleophiles produces different products.

Other application includes synthesis of wide variety of medicinal agents containing amine and amine derivatives with complete retention of stereochemistry during the reaction.
