Sulfonylation [SO/HSO]
Sulfonation of Benzene
The substitution of hydrogen atom on benzene ring by SO3H group via electrophilic aromatic substitution reaction is termed as Sulfonation reaction. This reaction is carried out by two methods. i) Benzene is refluxed with concentrated H2SO4 for several hours, ii) Benzene is reacted with fuming H2SO4 for 20 – 30 minutes. Fuming sulfuric acid is a solution containing 7% SO3 in H2SO4.

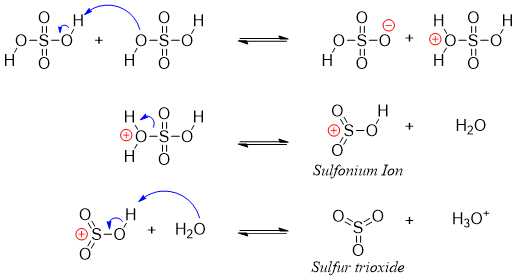
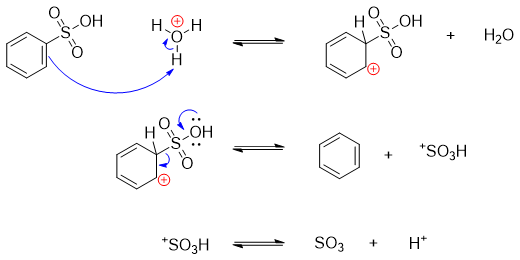
The active electrophile in sulfonation reaction is either SO3 or HSO3+ (sulfonium ion). The generation of electrophile is shown below.
The mechanisms of addition of electrophile via electrophilic aromatic substitution reaction is depicted below.
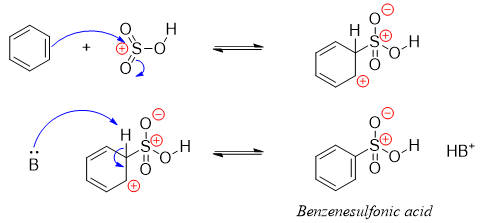
Above mechanism shows the addition of sulfonium electrophile.
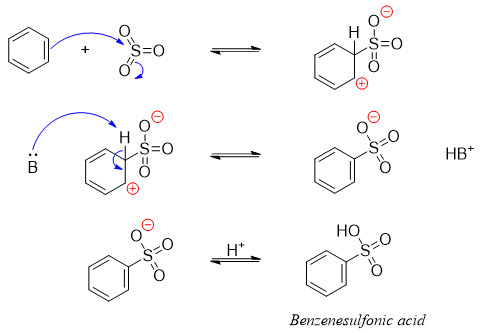
Above mechanism shows the addition of sulfur trioxide to the benzene ring.
The sulfonation reaction of benzene is highly reversible in nature. The process of sulfonation is favored in strong acidic conditions while, de-sulfonation is favored using dilute hot aqueous acids.
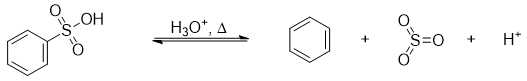
The mechanism of de-sulfonation reaction is shown below.
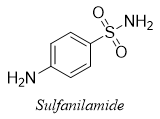
The process of sulfonation does not occur naturally. This process is employed to prepare sulfonyl derivatives having different useful applications. The first clinically used drugs used as antibiotics belonged to class of organic compounds called Sulfa drugs. For example, sulfanilamide.
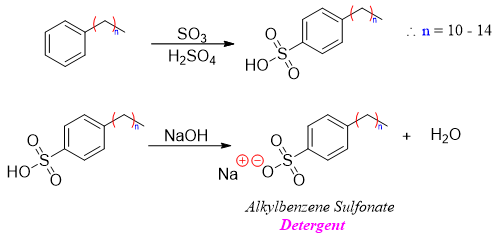
Another economical importance of sulfonation reaction is the use of alkyl sulfonates in the preparation of detergents. The alkyl group is normally a long chain of hydrocarbon consisting of 10 – 14 carbon atoms. The alkylbenzene sulfonic acid is treated with base to produce alkylbenzene sulfonate (detergent).