Friedel Crafts Alkylation
Friedel Crafts Alkylation
In 1887, two scientists named Charles Friedel and James Crafts treated amyl chloride (1-Chloropentane) with Aluminium chloride (AlCl3) in the presence of benzene (Scheme 1). The product isolated was amylbenzene. This was not only the first time Lewis acid was used in organic synthesis but also the first example of Friedel Crafts Alkylation.
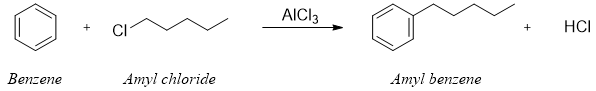
Scheme 1: Reaction between alkyl halide and benzene mediated by Lewis acid developed by Friedel and Crafts.
Over the years different Lewis acids were identified for accelerating the transformation in Friedel Crafts alkylation including BeCl2, BF3, SbCl5, TiCl4, or SnCl4.
In F. C. Alkylation reaction, alkyl halides are generally used as alkylating agents. Alkylating agents other than alkyl halides used are epoxides and enones in the presence of Brønsted–Lowry acids. The general F. C. Alkylation reaction is shown in scheme 2.
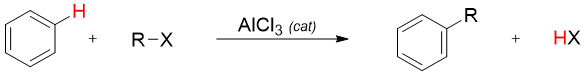
Scheme 2: General example of Friedel Crafts Alkylation
- C. Alkylation reaction is a type of electrophilic aromatic substitution reaction catalyzed by Lewis acids. As shown in scheme 2, the hydrogen atom on aromatic ring is substituted by alkyl group.
Mechanism:
The mechanism involves two steps i) The electrophile generated by the reaction of Lewis acid and alkyl halide adds to the benzene ring and ii) The base generated in situ removes the proton from the carbon bearing alkyl group to restore the aromaticity of benzene ring.
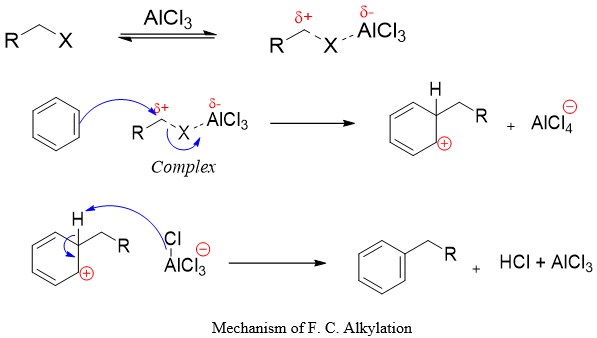
As shown in above mechanism, a carbocation like complex is involved when primary and possibly secondary alkyl halides are used. In case of tertiary alkyl halides, a free carbocations are formed as shown below.
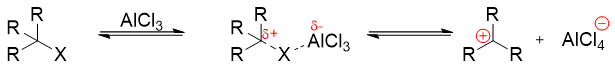
For large scale reactions, alkenes are usually used as the alkylating agents. The use of alkenes as alkylating agents requires protonation of alkenes to produce carbocations. The mechanism of use of alkene as alkylating agents is shown below.
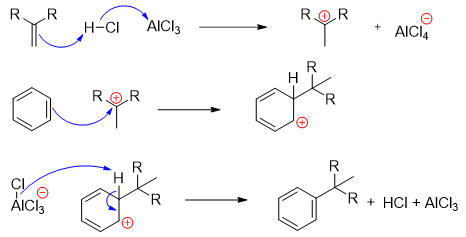
Due to substitution effect, the alkyl group present on benzene ring increases the electron density on benzene ring resulting in an increase in benzene ring reactivity. This increase in reactivity can lead to the formation of unwanted poly-substituted benzene. To avoid the reaction of alkylated benzene, a large excess of benzene is used. This ensures the formation of mono-substituted benzenes.
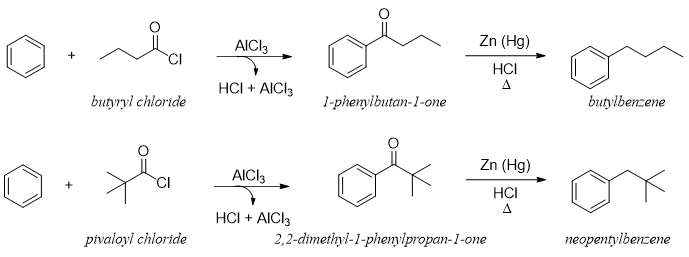
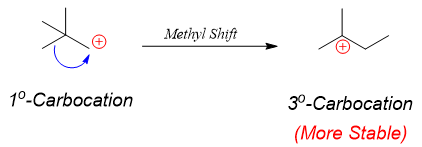
The rearrangement of carbocations is well known for attaining stability. When long chain primary alkyl halides are used in F. C. alkylation reactions the carbocations generated rearranges resulting in producing undesired products. For example, if butylbenzene is the desired product, it can not be synthesized by simply reacting 1-Chlorobenzene with benzene in the presence of Lewis acid because the primary carbocation can rearrange to produce more stable secondary carbocation.
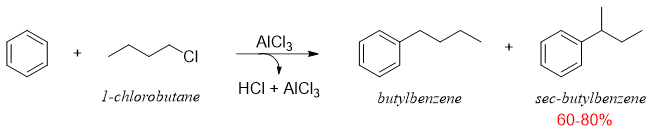
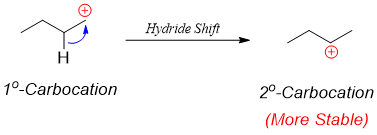
The rearrangement of primary carbocation to secondary carbocation is shown below.
Furthermore, the possibility of formation of tertiary carbocation will give the final product containing tertiary carbon atom. i.e.
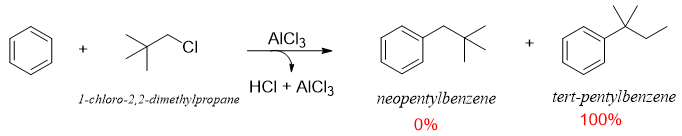
The rearrangement involving formation of tertiary carbocation is shown below.
To synthesize butylbenzene and neopentyl benzene as solo desired products F. C. Acylation reaction can be employed followed by reduction reactions (section F. C. Acylation).